1. Background
Chikungunya virus (CHIKV) is an alphavirus of the Togaviride family that transmits to humans by Aedes mosquitoes (Ae. albopictus and Ae. aegypti), which are spread across the tropical and subtropical regions around the world (1). This virus was first recognized in the 1950s in East Africa, and its transmission has been reported in Asia, Africa, Oceania, the Americas, and Europe (2, 3). The incubation period of CHIKV infection ranges from 1 to 12 days (7 days on average), and it has been reported that 3% - 25% of infections remain clinically asymptomatic (4, 5). The initial symptoms of the infection are generally divided into non-rheumatoid symptoms (rapid onset of high fever, maculopapular skin rash, and fatigue) and rheumatoid symptoms (myalgia, severe poly-arthralgia, joint stiffness, and stiffness in the extremities) (6, 7). In addition, the duration of CHIKV infection symptoms is classified into three phases, including acute stage (< 3 weeks), sub-acute stage (3 - 12 weeks), and chronic stage (> 12 weeks post-infection) (8). The acute phase can rarely be associated with severe manifestations. The most prevalent complications are multiple organ failure, sepsis, septic shock, and neurological complications (such as encephalitis and encephalopathy), all of which can be fatal (9-12). Despite these severe acute symptoms in some affected patients, most infected cases recover after a few days, and the rest of the patients may develop chronic symptoms, especially rheumatological and neurological complications (12).
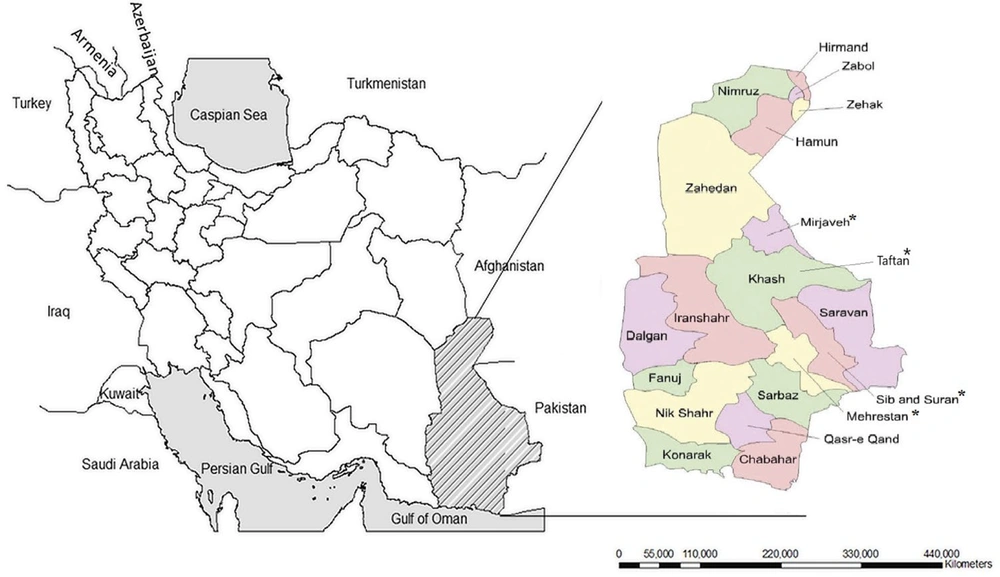
Our increased knowledge regarding the geographical spread of the CHIKV epidemics draws attention to the potentially severe infections caused by this virus as a re-emerging pathologic agent that can lead to a public health threat of global importance (13). The increasing volume of people's movement, including refugees and traffic of goods and animals between the borders, creates multiple opportunities for arbovirus infection (14). Most arbovirus cases in Iran were recognized in the southeast of the country near the border of Pakistan (15). Considering the proximity of Sistan and Baluchestan province to Pakistan, the continuous migration of the traders and residents on both sides, and the identification of CHIKV cases in the region (15), we conducted a comprehensive survey of the serological and clinical manifestations of CHIKV infections among the residents of 4 southeastern cities of Iran, including Taftan, Sib-and-Suran, Mehrestan, and Mirjaveh, which are the closest cities to the border of Pakistan (Figure 1).
2. Methods
2.1. Study Population
This cross-sectional study was performed in 4 cities in Sistan and Baluchestan province, from June 2020 to September 2020. According to the recent population census conducted in Iran, these cities had a total population of 245,207 people (45,327 in Mirjaveh, 85,089 in Sib-and-Suran, 44,176 in Taftan, and 70,579 in Mehrestan). In addition, the region's climate is semi-tropical and has been affected by monsoons, which can cause extensive rainfall and flooding every 3 - 5 years (15). Considering that the current research was an epidemiological study, the following formula was used to yield a representative sample for our population (N = 245,207, α = 0.05, p = 0.05; d = 0.03, n = 203) (15).
2.2. Data Collection
In this study, a questionnaire was used to collect sociodemographic and clinical data. The questionnaire included age, gender, and occupation. Furthermore, all participants were asked about their history of clinical manifestations, including fever, maculopapular skin rash, fatigue, and rheumatologic symptoms. In addition, 5 mL of blood was collected from each participant into a vacutainer tube and was allowed to clot at room temperature. Afterwards, serum was separated by centrifugation at 3000 rpm for 5 min, and sera aliquots of 1 mL were stored in a -20°C freezer until analysis. Each serum sample was screened for anti-CHIKV IgG antibodies using NOVATEC enzyme-linked immunosorbent assay (ELISA) kit (NOVATEC ELISA kit, Germany) based on standard protocol. Briefly, serum samples (diluted to 1:100) were added to microplates containing 50 µL of anti-CHIKV IgG and incubated at 37°C for 30 min. A calibrator, positive control, and negative control, provided by the manufacturer, were used. In the end, the optical density (OD) was evaluated by an ELISA reader (Anthos Labtec 2020 microplate reader, Austria).
2.3. Statistical Analysis
Descriptive statistics and the results of the serological tests were analyzed using IBM SPSS statistics version 26 (SPSS Inc., Chicago. Ill., USA). Chi-square (χ2) and independent t-tests were used to evaluate the association of demographic and clinical manifestations with CHIKV infection. P-values less than 0.05 were considered statistically significant.
2.4. Ethics Statement
Before participating in this study, each participant signed an informed consent form. In addition, this study was approved by the Research Ethics Committee of Zahedan University of Medical Sciences with the approval ID IR.ZAUMS.REC.1400.183.
3. Results
3.1. Geographical Location and Sociodemographic Characteristics
A total of 203 serum samples from the residents of 4 cities located in Sistan and Baluchestan provinces with relatively different weather conditions and close to the border of Pakistan were collected and analyzed. We observed that 143 cases were women and 60 were men. The number of participants based on residence was 71 cases (39.98%) from Sib-and-Suran, 59 (29.06%) from Mehrestan, 37 (18.23%) from Mirjaveh, and 36 (17.73%) from Taftan. The participants had an age range of 6 - 50 years, and the majority of them (27.5%) were 21 - 30 years old. There were also 48 cases (23.6%) in the age range of 31 - 40 years, 44 (21.6%) in 11 - 20 years, 32 (15.7%) in over 50 years, 21 (10.3%) in 41 - 50 years, and 2 (1%) in less than 10 years old. In terms of occupation, 123 cases (61%) were housewives, 35 (17%) were unemployed, 17 (8%) were independent contractors, 12 (6%) were employees, 8 (4%) were students, 3 (1%) were farmers, and 2 (1%) were children (Table 1).
| Variables | Anti-CHIKV IgG Antibodies | Total | P-Value | |
|---|---|---|---|---|
| Positive | Negative | |||
| City | 0.575 a | |||
| Mirjaveh | 0 | 37 | 37 | |
| Taftan | 0 | 36 | 36 | |
| Sib-and-Suran | 2 | 69 | 71 | |
| Mehrestan | 1 | 58 | 59 | |
| Age | 0.046 a, b | |||
| < 10 | 0 | 2 | 2 | |
| 11 - 20 | 0 | 44 | 44 | |
| 21 - 30 | 1 | 55 | 56 | |
| 31 - 40 | 0 | 48 | 48 | |
| 41 - 50 | 2 | 19 | 21 | |
| 50 < | 0 | 32 | 32 | |
| Gender | 0.209 a | |||
| Male | 2 | 58 | 60 | |
| Female | 1 | 141 | 143 | |
| Occupation | 0.001 a, c | |||
| Housewife | 1 | 122 | 123 | |
| Employee | 0 | 12 | 12 | |
| Farmer | 1 | 2 | 3 | |
| Independent contractors | 1 | 16 | 17 | |
| unemployed | 0 | 35 | 35 | |
| Student | 0 | 8 | 8 | |
| Children | 0 | 2 | 2 | |
| Clinical manifestation | < 0.001 a, c | |||
| None | 0 | 161 | 161 | |
| Fever | 1 | 16 | 17 | |
| Fever + arthralgia | 2 | 11 | 13 | |
| Hospitalization | 0 | 12 | 12 | |
a Fisher’s exact test.
b P < 0.05.
c P < 0.001.
According to the results of the self-reported questionnaire, 161 cases (79.31%) had no history of clinical symptoms, 17 (8.37%) had a history of fever or several episodes of fever, 13 (6.4%) reported a history of fever and arthralgia, and 12 (5.91%) a history of recent hospitalization (Table 1).
3.2. Serological Examination of Anti-CHIKV Antibodies
Out of 203 samples examined by ELISA, 3 samples (1.48%) were positive for anti-CHIKV IgG antibodies, two of whom were women and one was a man. Two of these people lived in Sib-and-Suran, and one lived in Mehrestan. We did not find any positive cases from Taftan and Mirjaveh. Two of the positive cases were aged 41 - 50 years, and the other one was in the age group of 21 - 30 years. In terms of occupation, one of the positive cases was a housewife, one was a farmer, and the other person was self-employed. One of these people reported a history of fever, and the other two reported a history of fever and arthralgia (Table 1).
4. Discussion
Chikungunya virus is one of the most serious mosquito-borne pathogens that has been spreading recently and can have devastating human morbidity in many regions of the world. Compared to some other arthropod-borne infectious agents, CHIKV has been relatively understudied and is now considered an important yet neglected tropical disease. In the past, most CHIKV outbreaks occurred in countries in Africa, Europe, Asia, and the Indian and Pacific Oceans, mainly due to the geographical spread of virus-carrying vectors. However, at the end of 2013, the first case of the local transmission of CHIKV was diagnosed in the Americas. Subsequently, the local transmission of the disease was reported by the World Health Organization in 45 other countries, which was much more than expected (16-19). Therefore, this virus has recently caused widespread epidemics in many tropical and subtropical nations.
In November 2016, a large epidemic occurred in Karachi, Pakistan, which is located in the neighborhood of our regions of study, and approximately 30,000 people were infected with CHIKV (20). Due to the proximity of Sistan and Baluchestan province to Pakistan, ongoing trades, nomads on both sides of the border, and the tropical and subtropical climate of this region, it is necessary to continuously monitor the possible spread of the virus in this region. Moreover, the presence of febrile infections, such as malaria, brucellosis, dengue fever, typhoid fever, and Crimean-Congo hemorrhagic fever (CCHF), in this region makes it necessary to differentiate chikungunya infection from these infections.
In our study, we had only 3 positive samples out of 203, which is equal to 1.48% of all specimens. In 2019, Pouriayevali et al. examined 159 samples from febrile patients suspected of CCHF in Boali Hospital, Zahedan, Iran, for CHIKV infection. The results showed that approximately 25% of samples were positive for CHIKV genome or antibodies (15). In another study, Tavakoli et al. investigated the presence of dengue virus (DENV) and chikungunya IgM antibodies in patients with rash and fever who were negative for measles and rubella. The findings of this study showed that DENV IgM and CHIKV IgM were present in 16% and 6% of these patients, respectively (21). These studies and our findings confirm the existence of CHIKV in Sistan and Baluchestan province. However, given that our study was completed on non-hospitalized people, it is not possible to compare the findings with those of Tavakoli et al. (21).
The four cities our patients were from are quite different in geographical and climate conditions. Our three positive patients were from the two cities of Sib-and-Suran and Mehrestan, both of which are located near the border with Pakistan and share a similar climate. We expected positive results prior to testing due to the occurring trades and the constant movement of the people of this region to and from Pakistan. We also examined two other cities, Mirjaveh and Taftan. Mirjaveh is further from the border compared to the last two cities and is located more centrally within the province. However, in terms of location and climate, it is similar to the other two cities. We did not have a positive sample from this city. It shows that besides the tropical climate and proximity to Pakistan, other factors, such as routine travel, also increase infection spread. Our last city was Taftan, which is located near Taftan Mountain. Taftan is relatively different from the other three cities, with a cooler climate and a longer distance from the Pakistan border. We found no positive samples in this city either.
We also examined the subjects in terms of age, gender, and occupation. As shown in Table 1, two of the positive cases were aged 41 - 50 years, and one case was in the age group of 21 - 30 years old. Statistical analyses in a meta-analysis in 2020 showed that older age (> 28 years) was associated with infection with arbovirus seropositivity in infected regions (22). Similarly, a higher tendency of arbovirus infections has been associated with older age (22, 23). The predominance of the female population renders our results unreliable and fairly debatable. The positive cases in our study were from three different occupational groups (housewife, farmer, and self-employed). Galatas et al. in 2016 showed that having an indoor occupation was associated with lower odds of infection in comparison with outdoor occupations (24). Kumar et al. also demonstrated that the prevalence of viral Aedes albopictus infections was higher in individuals engaged in plantation activities due to higher exposure to the bites of Aedes mosquito (25). In another study, the prevalence of CHIKV was significantly higher in farming occupations compared to other jobs (22). Therefore, the findings of this study highlight the increasing rate of infection in farmers who work outdoors and are in contact with mosquito breeding areas (due to the short flight range of mosquitos) without any anti-adult mosquito measures.
Based on the self-report of positive cases in our study, the predominant clinical history in these people was either fever or fever and arthralgia. Similar to our study, previous studies also reported that arthralgia, especially in larger joints, was one of the main clinical manifestations of CHIKV (26). In another study, Goupil and Mores declared an apparent relationship between CHIKV infection and acute arthralgia. Nevertheless, it seems that chronic features are associated with viral persistence, autoimmune disease, and exacerbation of pre-existing joint disease (27). In addition, Kawle et al. observed a higher prevalence of clinical manifestations, such as small joint pain, neck stiffness, fever, large joint pain, and rash (on knees, feet, fingers, and palms), among individuals infected with CHIKV (28).
4.1. Conclusions
The existence of CHIKV in the southeast of Iran, confirmed by seroprevalence analyses, indicates that diverse factors may contribute to the spread of CHIKV, including climate and proximity to Pakistan, as well as trade interactions and constant movement. Therefore, awareness of viral presence and its potential spreading routes can assist in preventing future outbreaks and virus spread.