1. Context
Coronaviruses (CoVs) are zoonotic viruses belonging to the single-stranded RNA viruses family. Coronaviruses can infect a variety of animals and are associated with a range of diseases, including respiratory, cardiovascular, enteric, hematologic, and neurologic disorders. They frequently cause a range of symptoms, including respiratory and gastrointestinal tract diseases, common cold, pneumonia with symptoms such as cough, dyspnea, tachypnea, and chest pain, bronchitis, and severe acute respiratory distress syndrome (ARDS) with arterial hypoxemia and dyspnea. Other symptoms can include coagulopathy, multi-organ failure, cystic fibrosis, asthma, chronic obstructive pulmonary disease, and even death in humans (1).
Coronaviruses were previously known to primarily cause lower respiratory tract infections, but it is now understood that they can affect multiple systems. Several hematological abnormalities have been observed, including changes in hemoglobin, platelets, white blood cells, and coagulation/fibrinolytic factors. Thrombocytopenia, lymphopenia, and elevated D-dimer levels are common hematological abnormalities in coronavirus disease 2019 (COVID-19). These changes are notably more frequent and pronounced in patients with severe COVID-19 and could be used as biomarkers to identify individuals requiring hospitalization and intensive care. Monitoring coagulation abnormalities closely and taking appropriate actions to prevent or mitigate their adverse effects is crucial (2). Based on these observations, we have decided to examine the abnormalities related to hematologic biomarkers in patients with COVID-19.
2. Evidence Acquisition
COVID-19, caused by the SARS-CoV-2 virus, can significantly impact the hematologic system, leading to various abnormalities that serve as valuable biomarkers for disease severity, prognosis, and therapeutic monitoring. In this narrative review, we identify hematologic biomarker abnormalities in patients with COVID-19 and discuss their clinical significance based on the most recent information.
3. Results
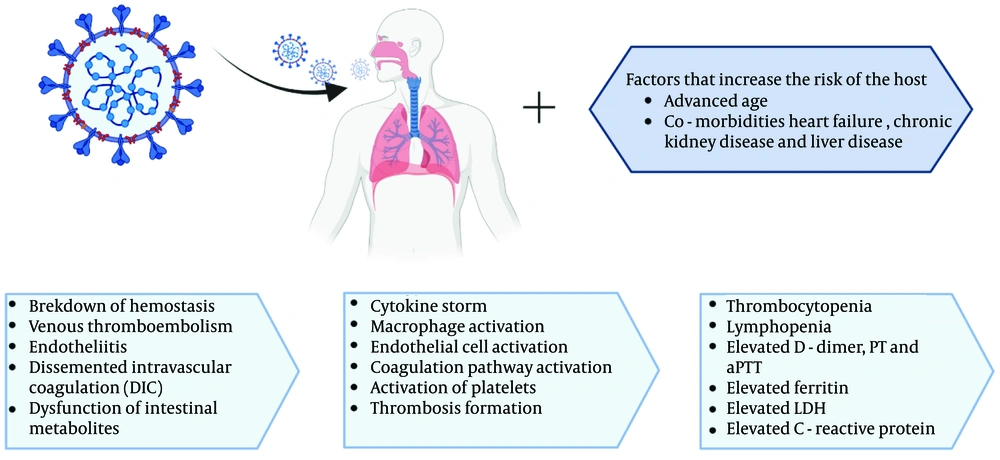
COVID-19 is classified as an infectious-inflammatory disease that primarily affects the lungs. According to a retrospective analysis, dry cough (60%) and fever (89%) were the most prevalent clinical symptoms (3). Recently, multi-organ involvement with various injury pathways has been identified. Disruptions in the balance between an effective antiviral response and an uncontrolled immune response are key determinants of the severity of COVID-19 progression. Comorbidities have been correlated with hematological, immunological, and biochemical parameters. When CoVs infect endothelial and monocyte cells, a complex cytokine storm and eventual intravascular thrombosis can result (1, 2). In this context, we examined some of the most prevalent hematologic abnormalities in patients with COVID-19.
3.1. Hemoglobin Variants and Diabetic Patients
Diabetes is a risk factor for the development and prognosis of COVID-19. There is limited information about the relationship between inflammation, glycated hemoglobin (HbA1c) levels, and the prognosis of COVID-19 patients. In the study by Wang et al. (4), 73% of the patients had comorbid conditions, including hypertension and diabetes, followed by cardiovascular, cerebrovascular, and chronic kidney diseases. At the time of admission, oxygen saturation (SaO2) varied between 59% and 99%. Significant differences were observed in parameters such as C-reactive protein (CRP), ESR, IL-6 levels, SaO2, serum ferritin, and fibrinogen (FBG). Glycated hemoglobin levels had a linear negative correlation with SaO2, while serum ferritin, FBG, CRP, and ESR levels had a linear positive correlation with HbA1c.
In COVID-19 patients, high HbA1c levels were associated with lower arterial SaO2, inflammation, and hypercoagulability, and patients with diabetes had a higher mortality rate. Therefore, determining HbA1c levels after hospital admission could help evaluate the prognosis of COVID-19 patients, as well as assess hypercoagulability and inflammation (4). Additionally, in patients with diabetes who developed ARDS, levels of D-dimer and lactate dehydrogenase were increased (5).
Hemoglobinopathy, hypoxia, and iron overload in cells could also play a role. Different studies have identified two possible pathophysiological mechanisms: One involves the interaction of SARS-CoV-2 with the hemoglobin molecule via the CD26 or CD147 receptors found on erythrocytes and/or blood cell precursors. The other mechanism involves a viral spike protein that mimics hepcidin, resulting in the induction of ferroportin blockage. Cavezzi et al. emphasized the pathological metabolic pathways resulting from iron metabolism dysregulation and hemoglobin denaturation. The following factors contribute to decreased hemoglobin function: (a) iron overload in cells and tissues (hyperferritinemia); (b) hypoxia and systemic hypoxia; (c) release of free toxic heme; (d) decreased nitric oxide levels; (e) activation of the clotting cascade; (f) ferroptosis with lipoperoxidation and oxidative stress; and (g) mitochondrial apoptosis and degeneration (6).
Several clinical syndromes have been reported, including arterial vasoconstriction, altered alveolar-capillary barriers, arteriovenous thromboembolism, endothelitis, vasospastic acrosyndrome, and sideroblastic-like anemia. A more thorough diagnosis and treatment plan for COVID-19 was suggested, including potential adjuvant treatments for hemoglobin dysfunction, iron overload, and generalized hypoxia (7, 8).
Diabetes is a risk factor for COVID-19 development, but there is limited information on the relationship between HbA1c levels, inflammation, and the prognosis of COVID-19 patients. Some inflammatory markers, such as ESR, CRP, serum ferritin, and FBG, were positively correlated with HbA1c levels, whereas SaO2 was negatively correlated with HbA1c levels. Interestingly, in patients with elevated HbA1c but without diabetes, levels of inflammatory markers and FBG were also significantly increased. In COVID-19 patients, elevated HbA1c was associated with inflammation, low SaO2, and hypercoagulability. It has also been reported that the mortality rate is higher in patients with diabetes. Based on previous studies, measuring HbA1c levels is useful for assessing hypercoagulability and inflammation in the prognosis of COVID-19 patients after hospital admission (4, 9, 10). Low hemoglobin (Hb) levels significantly predicted ICU admission and mortality (P = 0.004) (11).
3.2. Coagulation Abnormalities (Prothrombin Time, Partial Thromboplastin Time) and Platelet Reactivity
Patients with COVID-19 must receive prophylactic anticoagulation due to the disease's impact on the coagulation system, which is associated with high rates of morbidity and mortality. Pulmonary microthromboses and pulmonary embolism can result from arterial and venous thromboses. Identifying these complications and promptly initiating therapeutic anticoagulation is crucial for effective management. Although less common, virus-induced disseminated intravascular coagulation (DIC) shares some similarities with traditional DIC. Poor outcomes are associated with elevated levels of hematologic biomarkers such as D-dimer, C-reactive protein, lactate dehydrogenase, and ferritin. Understanding the pathophysiology of COVID-19 and identifying factors linked to poor prognosis are essential for improving patient outcomes (3).
The high frequency of thrombosis in COVID-19 is a concerning feature, particularly among patients admitted to the intensive care unit due to respiratory complications. Thrombotic events are widespread and include deep vein thrombosis (DVT), arterial thrombotic events, in situ pulmonary arterial thrombosis (PAT), and pulmonary embolism (PE). There have also been reports of unusual thrombotic events such as mesenteric artery and vein thrombosis, cerebral venous sinus thrombosis, neutrophilia, lymphopenia, thrombocytosis, and mild thrombocytopenia. Elevated levels of prothrombin time (PT), activated partial thromboplastin time (aPTT), von Willebrand factor, fibrin D-dimer, and hyperfibrinogenemia are among the hematological abnormalities observed in COVID-19 patients. Many of these abnormal hematological parameters are indicative of a poor prognosis, including a higher likelihood of developing severe respiratory disease and death, even at the time of initial hospitalization. Some studies have reported progression to diffuse intravascular coagulation in fatal COVID-19 cases. It has been demonstrated that, during the prevention and treatment of COVID-19 thrombosis using low molecular-weight heparin or unfractionated heparin, vigilance for heparin-induced thrombocytopenia (HIT) is necessary (12). Elevated D-dimer levels have also been reported among COVID-19 patients. There is some evidence linking high rates of venous thromboembolism (VTE) and DIC with COVID-19. Parameters such as procalcitonin, fibrinogen, ESR, ferritin, and CRP are typically higher in patients with thrombotic complications compared to those without. Clinically, while DIC rarely presents with reduced fibrinogen and thrombocytopenia, it is associated with significant bleeding manifestations. Therefore, to determine the potential benefit of intensified anticoagulant prophylaxis in COVID-19 patients, randomized trials are needed, given the observed bleeding rates (13, 14).
It was also discovered that COVID-19 was associated with rates of thrombosis and bleeding comparable to those observed in hospitalized patients with similar levels of critical illness. Elevated D-dimer levels at the time of initial presentation indicated a higher likelihood of thrombotic complications, severe illness, and death. In addition to D-dimer, inflammatory markers, rather than coagulation parameters, were more frequently associated with thrombosis (12).
3.3. Leukocyte Morphology and Count
White blood cell levels were found to be significantly correlated with mortality. Using the second quartile threshold (> 6.16 × 109/L), regression analysis revealed a significant correlation between WBC levels and death. Kaplan-Meier survival analysis indicated a significantly lower cumulative survival rate for patients with leukopenia (P = 0.001) below 6.16 × 109/L. Liu et al. (15) reported that about 80% of patients had either a normal or reduced number of white blood cells, with 72.3% exhibiting lymphocytopenia. Conversely, all patients with negative PCR results had mostly normal peripheral white blood cell counts. However, patients with higher WBC counts were at greater risk of mortality (15-17).
It was also demonstrated that neutrophil (NEU) count, lactate dehydrogenase (LDH), CRP, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and serum urea levels were all significantly higher in patients with positive reverse transcriptase PCR (RT-PCR) results. Additionally, these patients had lower serum albumin levels and WBC counts compared to others. C-reactive protein, ALT, LDH, NEU count, and urea all demonstrated excellent accuracy in predicting positive RT-PCR cases for COVID-19 (18).
It has been reported that leukopenia, lymphopenia, high-sensitivity CRP (hsCRP), ESR, and fibrinogen concentrations are elevated in COVID-19 patients. Additionally, after treatment, levels of AST, hsCRP, and LDH decreased, while the numbers of RBCs, platelets, and pre-albumin increased significantly (19).
In severe and critical cases, the total number of lymphocytes, CD3, and CD4 T-cell levels were reduced in patients who developed ARDS. However, IL-6 levels were high in both mild and severe cases. Regardless of disease severity or the presence of comorbid conditions, ESR, WBC count, and CRP levels increased. In patients with severe and critical COVID-19, procalcitonin levels varied, showing either an increase or decrease, but CD3+, CD4+ T cells, and the total lymphocyte count were found to be low. Elevated levels of CRP, ESR, and IL-6 were noted. Understanding the inflammatory response in COVID-19 patients is essential for developing better therapeutic and management approaches (2, 20).
Hemocytometric changes in COVID-19 infection, both early and throughout the course of the disease, have been examined in numerous studies. The most common abnormalities reported include lymphopenia and an increased neutrophil/lymphocyte ratio. These alterations become more pronounced over time, especially in patients with advanced disease (21).
It has been demonstrated that patients with severe COVID-19 exhibit lymphopenia and decreased levels of B cells, CD4+ T cells, CD8+ T cells, and natural killer (NK) cells compared to patients with mild COVID-19. Notably, CD8+ T cells and the CD4+/CD8+ ratio showed a significant correlation with the inflammatory status of COVID-19. In non-responsive cases, no significant changes were observed in these subsets. Multivariate analysis identified an increased CD4+/CD8+ ratio and a decrease in CD8+ T cells and B cells post-treatment as independent predictors. Additionally, alterations in peripheral lymphocyte subsets were associated with COVID-19 severity and treatment effectiveness, with CD8+ T cells frequently serving as an independent predictor (22). On the other hand, the average number of neutrophils was significantly higher in patients who died (P = 0.032) (11).
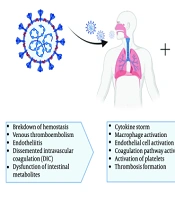
Granulocyte colony-stimulating factors (G-CSFs) promote cytokine production and neutrophil proliferation. Neutropenia has frequently been treated with G-CSFs, despite limited efficacy data. A relationship has been reported between respiratory distress and neutropenia, but not with G-CSFs. In most clinical settings, G-CSFs have a history of being safe and well-tolerated. However, there is limited information about the effects of G-CSFs in severe inflammatory conditions like COVID-19. Taha et al. reported a case where COVID-19 with neutropenia deteriorated quickly after receiving G-CSF. They observed a faster recovery from neutropenia following G-CSF administration. The rapid recovery from neutropenia and the active inflammatory response in COVID-19 patients present a significant challenge regarding the positive effects of G-CSF in these patients (23). Figure 1 shows the mechanisms of hematological abnormalities in COVID-19 (24).
Main laboratory changes in patients with an unfavorable evolution of SARS-CoV-2 infection (24)
3.4. Immunological Markers
In patients infected with COVID-19, ω-3 polyunsaturated fatty acids (n3-PUFAs) can have beneficial effects on the immune system. It has been established that ω-3 PUFA supplementation may improve clinical outcomes in patients with COVID-19, including promising effects on acidosis and renal function. ω-3 PUFA supplementation resulted in significantly higher levels of arterial pH and HCO3, and lower levels of BUN, Cr, and K. However, ω-3 PUFA supplementation did not significantly affect blood glucose, Ca, P, Na, HCT, mean arterial blood pressure (MAP), SaO2, PO2, PCO2, WBCs, Glasgow Coma Scale (GCS), Hb, platelet (Plt) count, PTT, or albumin. Despite this, some parameters related to respiratory and kidney function in patients with acute COVID-19 showed improvement with ω-3 supplementation. Additionally, ω-3 supplementation may potentially increase lymphocyte count and GCS. It has been reported that patients with severe COVID-19, possibly due to microcirculation dysfunction, experience severe metabolic and respiratory acidosis (25-29).
Cellular metabolism has been found to be activated during viral infection and the replication of SARS-CoV-2. A serine-threonine kinase that contributes to cell proliferation is mammalian targets of rapamycin (mTOR). During viral replication, the mTOR pathway facilitates the efficient synthesis of ribosomes and proteins by activating downstream genes such as the eukaryotic translational initiation factor 4E-binding protein 1 (4-E-BP1) and the ribosomal protein S6 kinase beta 1 (S6K1). Mammalian targets of rapamycin also enables the activation of type-I interferon (IFN) genes by mediating the association of the adapter protein MyD88 and the interferon regulatory factor (IRF-7) in plasmacytoid dendritic cells (pDCs). Additionally, viruses can deactivate the IFN pathway by disrupting the IRF-7-mediated activation of IFN-gene transcription. Consequently, mTOR inhibitors could help prevent viral infection and replication in their early stages. Intriguingly, viral survival in host cells may be increased because the virus-encoded E3 ubiquitin ligase ring-finger and CHY zinc-finger domain-containing 1 (RCHY1) degrades the important tumor-suppressor protein p53. Therefore, microRNAs can act as p53 activators and mTOR inhibitors, targeting the 3'-UTR of mTOR RPS6KB1. Both mechanisms may effectively inhibit viral replication in human respiratory tract and lung cells (30).
In response to the coronavirus, host immune responses vigorously attempt to combat the infection using all available cells and cytokines. Chronic SARS-CoV-2 infections can exhaust T cells and natural killer cells, leading to lymphopenia and a reduced cell count. The inability to clear the infected organs causes the immune system to become hyperinflamed, resulting in the release of excessive inflammatory cytokines to compensate for the depleted immune system and lower lymphocyte counts. This results in cytokine storm syndrome (31).
Several indices in patients with acute COVID-19 were significantly higher compared to those in mild cases (P ≤ 0.05), including neutrophil count, eosinophil count (EOs), WBC count, platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), red blood cell distribution width-standard deviation (RDW-SD), lymphocyte count (Lym), and high fluorescent cell percent (HFCP). Assessing combined NLR and RDW-SD parameters can be a useful indicator to help clinicians anticipate the severity of COVID-19 in patients (32).
A higher NLR was significantly associated with mortality. Non-surviving patients had notably lower Plt counts (P = 0.023). Compared to survivors, non-survivors had significantly lower Plt and Hb counts (P = 0.001 for both). The PLR was also significantly higher among non-survivors (P = 0.034) and correlated with mortality. It has been suggested that hematological laboratory parameters are crucial for developing effective diagnosis and treatment plans for infectious diseases. In hospitalized COVID-19 patients, parameters such as PLR, NLR, Hb, low Plt, prolonged PT, and elevated d-dimer, along with inflammatory markers, can serve as hematologic predictors of severe outcomes (31).
3.5. XGBoost Algorithm Scoring
According to the feature importance scoring of the XGBoost algorithm (a gradient-boosting decision tree method used globally by data scientists and researchers to optimize machine-learning models), MCHC, INR, albumin, EOs, and PT were identified as the five most useful routine blood parameters for diagnosing COVID-19.
Changes in blood parameters in severe COVID-19 patients resemble those seen in bacterial infections more than in viral infections, as indicated by T-distributed stochastic neighbor embedding (t-SNE) visualization. The t-SNE technique is a dimensionality reduction method that helps visualize high-dimensional data. The reported diagnostic accuracy of this approach is likely supportive of RT-PCR and chest CT studies, if not at least comparable to them. Patients exhibiting symptoms such as fever, coughing, and myalgia could have their preliminary routine blood tests evaluated using this diagnostic tool. Those with positive COVID-19 indications would then undergo standard RT-PCR tests for confirmation. The outcomes of this approach are claimed to significantly aid in the diagnosis of COVID-19 (33).
3.6. Age-Dependent Differences in the COVID-19 Intensity
There are several studies on age-dependent differences in COVID-19 intensity. Explanations for the disparity between the intensity of COVID-19 in children and adults include risk factors for adults and protective factors for children. It has been shown that many parameters are age-dependent, including:
- Changes in clotting function
- Endothelial damage
- Higher density, greater affinity, and different distribution of transmembrane serine protease 2 and angiotensin-converting enzyme 2 receptors
- Pre-existing coronavirus antibodies (including antibody-dependent enhancement) and T cells
- Immunosenescence and inflammation, including the effects of chronic cytomegalovirus infection
- A higher prevalence of comorbidities associated with diabetes
Children may be protected by factors such as different microbiota, distinct innate and adaptive immune systems, pre-existing immunity to coronaviruses, more frequent recurrent infections, higher levels of melatonin, less intense SARS-CoV-2 exposure, and protective off-target effects of live vaccines (34, 35).
It has been demonstrated that both PLR and NLR are readily available measurements linked to inflammation and prognosis in various conditions. The relationship between PLR and NLR and the severity of COVID-19 has been identified using available data. PLR and NLR can serve as separate prognostic markers of disease severity (36).
3.7. Abnormal Red Blood Cells
Red blood cell distribution width, which has previously been shown to be a helpful prognostic parameter in various serious diseases and infectious illnesses, was examined by Henry et al. (35) in patients with COVID-19. As COVID-19 severity increased, a progressive rise in RDW was observed. In multivariate analysis, elevated RDW was associated with 9-fold and 16-fold increased odds of severe COVID-19 and acute kidney injury (AKI), respectively. Based on these findings, RDW should be included in the routine laboratory assessment and monitoring of COVID-19 (35).
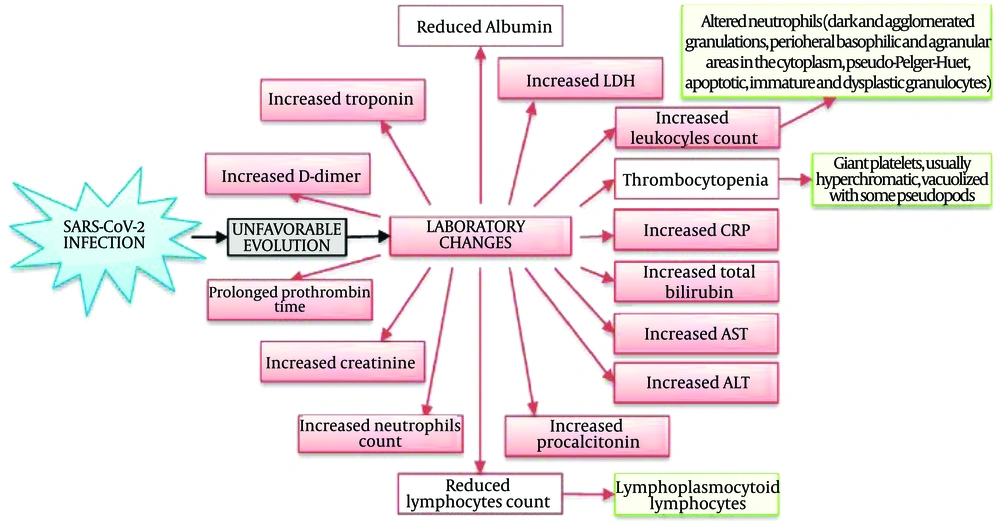
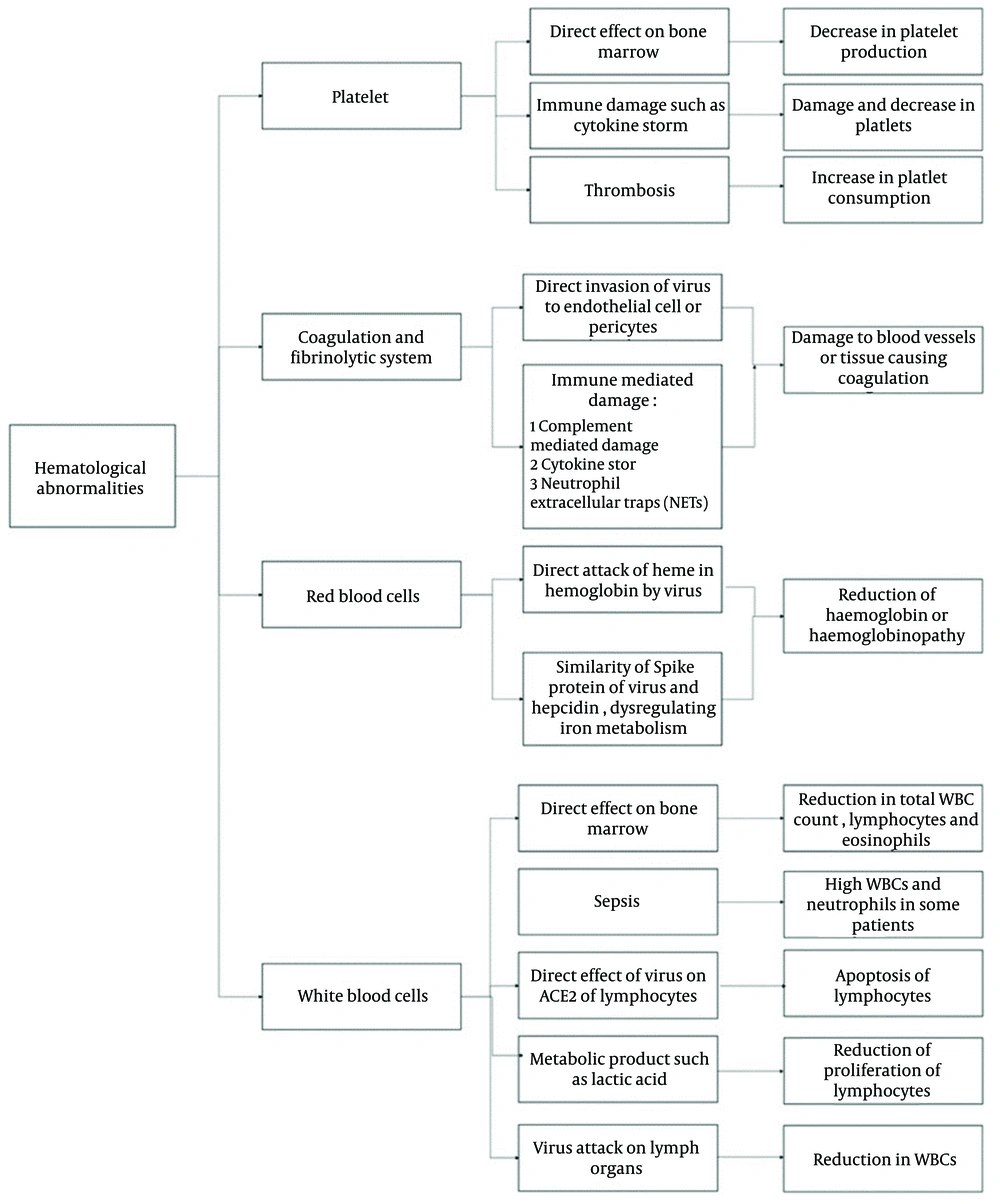
Figure 2 illustrates COVID-19-associated hematologic manifestations, including changes in blood cell counts (lymphopenia, neutrophilia, thrombocytopenia), coagulation disorders (DIC, increased risk of VTE), and abnormal levels of D-dimer, CRP, LDH, and clotting factors. These issues highlight the complex interplay between the virus, immune response, and blood clotting (23). Figure 3 presents the mechanisms of hematological abnormalities in COVID-19 (36).
Coronavirus disease 2019 (COVID-19)-associated hematologic manifestations in patients (23)
Mechanisms of hematological abnormalities in coronavirus disease 2019 (COVID-19) (36)
4. Conclusions
According to our study, several biomarkers for predicting severe and fatal COVID-19 were identified, which may potentially aid in risk stratification models. These findings could delineate a new etiological pathway for future exploration. Therefore, clinicians should be aware of the potential paraclinical indicators in COVID-19 patients to avoid neglecting or overdiagnosing cases.