1. Background
Atrioventricular septal defect (AVSD) is a progressive chronic inflammatory process that can thicken vascular walls (1, 2). Cardiovascular diseases (CVD) are primarily caused by AVSD, the leading cause of global mortality, projected to affect approximately 23.4 million individuals (3). Several risk factors, including hypertension, hypercholesterolemia, diabetes, extreme obesity, smoking, physical inactivity, and chronic bacterial infections, have been linked to the development of AVSD (4). Recent studies over the past two decades have indicated that microorganisms and conventional risk factors play a role in the initiation and progression of atherosclerotic plaques (4-7). Notably, periodontal pathogens such as Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) and Porphyromonas gingivalis (P. gingivalis) have been strongly associated with coronary artery disease and myocardial infarction (8). P. gingivalis, the primary pathogen in chronic periodontitis, contributes to the pathogenesis of atherosclerosis through the presence of lipopolysaccharides (LPS) as endotoxins and its ability to translocate into systemic blood vessels from the oral cavity (8). The DNA of P. gingivalis is commonly found in atheromatous plaques of individuals with periodontal diseases (9).
Periodontitis is a chronic asymptomatic inflammatory disease characterized by a slow progression. It occurs following the colonization of gram-negative anaerobic bacteria in the tooth or subgingival margin, resulting in damage to the supporting tissues (3, 5, 10). The chronic inflammatory responses triggered by periodontitis have been considered potential risk factors for AVSD (7). Studies have shown that patients with periodontitis and ulcerated epithelium in the pocket wall experience transient bacteremia (7, 11, 12).
Among the numerous bacterial species in human subgingival plaque, P. gingivalis is the primary causative agent of chronic periodontitis. This bacterium produces various virulence factors that modulate the host's inflammatory response and contribute to the destruction of periodontal tissues (13). Evidence suggests that periodontal pathogens are involved, either directly or indirectly, in platelet accumulation, increased levels of low-density cholesterol, lipoprotein deposition in the vascular wall, endothelial damage in the heart and carotid arteries, and elevated levels of inflammatory mediators in the blood and tissues (4). Persistent low-level bacteremia associated with chronic periodontitis can lead to the invasion of bacteria into endothelial cells, causing endothelial dysfunction and triggering pro-inflammatory and pro-atherogenic responses. Periodontal pathogens activate pro-inflammatory cytokines and chemokines, forming foam cells, which is an early stage in the development of atherosclerosis (14).
Numerous studies have emphasized the critical role of anaerobic bacteria, including P. gingivalis and A. actinomycetemcomitans, in developing CVD. For instance, Gaetti-Jardim et al. found a significant association between P. gingivalis in subgingival plaques of patients with periodontitis and atherosclerotic plaques in the same individuals (4). While some studies have reported a similar correlation (1, 3), others have not found a significant relationship between P. gingivalis in subgingival and atherosclerotic plaques in patients with periodontitis (9, 15).
2. Objectives
Given the conflicting findings regarding the potential involvement of P. gingivalis in coronary artery diseases (CAD), which continue to be the leading cause of mortality worldwide (2-4, 16), the present study aimed to investigate the presence of P. gingivalis DNA in autopsy samples of subgingival and atherosclerotic plaque obtained from corpses transferred to the Tehran Forensic Medicine Organization.
3. Methods
3.1. Patients
This descriptive cross-sectional correlational study was conducted on deceased individuals transferred to the Forensic Medicine Organization to determine the cause of death. All procedures were performed following the ethical guidelines laid down by the Declaration of Helsinki. The study population consisted of 16 (64%) males and 9 (36%) females, with an average age of 56. Inclusion criteria required individuals to have at least ten teeth and two pockets with a depth exceeding 5 mm. Additionally, these individuals were diagnosed with coronary atherosclerotic plaques during the autopsy, performed within 6 hours postmortem. Comprehensive data, including systemic conditions, age, weight, height, history of drug addiction, and smoking habits, were extracted from the cadavers' records. Before commencing the study, ethical approvals and permissions were obtained from the appropriate authorities at the Forensic Medicine Organization. Written consent was obtained from the families of the cadavers involved in this research study.
3.2. Sample Collection
The autopsies were performed by experienced forensic experts who meticulously examined the condition of the coronary arteries. Identification of coronary arteries with atherosclerotic plaques was confirmed. Subsequently, probing of all tooth surfaces was conducted using a Williams probe. Subgingival plaque samples were collected from the deepest regions of pockets exceeding 5 mm, employing sterile paper points. These samples were then carefully transferred to Eppendorf tubes containing trypticase soy broth (TSB) medium. Forensic experts excised atherosclerotic plaques measuring at least 1 × 1 mm and promptly placed them in separate Eppendorf tubes containing TSB. Each of the 25 eligible cadavers contributed one subgingival plaque sample and one atherosclerotic plaque sample. All collected samples were promptly stored at -20°C and transported to the Microbiology Department of Shahid Beheshti University of Medical Sciences to analyze P. gingivalis DNA using the real-time polymerase chain reaction (PCR) method.
3.3. DNA Extraction and Real-time PCR
Bacterial DNA extraction was performed on all plaque samples using the high-pure PCR template preparation kit (Roche, Germany, Lot No. 10362400), following the manufacturer's instructions. The Nanodrop instrument (WPA Biowave II Nanospectrophotometer, USA) determined the total DNA concentration. Qualitative real-time PCR was conducted using the BIOFACT Sybr Green master mix (CAT. NO.: PR901638) on a real-time PCR system (QuantStudio™ 6 Flex Real-Time PCR System, Thermo Fisher Scientific). The real-time PCR reactions were prepared in a final volume of 20 μL, comprising 2 µL of extracted genomic DNA as the template, 10 μL of master mix, and 0.4 M each of the forward and reverse primers. The primer sequences used were as follows: 3'-AGGCAGCTTGCCATACTGCG-5' (forward) and 3'-ACTGTTAGCAACTACCGATGT-5' (reverse) (17). PCR-grade water was utilized as a negative control instead of template DNA.
The conditions for the qualitative real-time PCR were as follows: An initial denaturation step at 95°C for 10 minutes, followed by 45 cycles of denaturation at 95°C for 15 seconds, annealing at 50°C for 30 seconds, and extension at 72°C for 30 seconds. The accumulation of PCR products at each cycle was monitored by measuring the increase in fluorescence intensity of the dsDNA-binding Sybr Green.
3.4. Data Analysis
The quantitative variables were described using mean, standard deviation, minimum, and maximum, whereas qualitative variables were explained in percentages. McNemar's test was used for pairwise comparison of PCR results in two samples. The results' correlation and the agreement's Kappa coefficient were calculated in the whole group and subgroups. A P-value < 0.05 was considered statistically significant.
4. Results
This research aimed to investigate the presence of P. gingivalis DNA in subgingival and atherosclerotic plaques collected from 25 eligible corpses. Among the examined corpses, three individuals (12%) were identified as smokers, while seven individuals (28%) had a history of alcohol or drug addiction.
The molecular tests revealed that 40% (10 samples) of subgingival plaques and 16% (4 samples) of atherosclerotic plaques tested positive for P. gingivalis DNA. Among the 21 negative samples of atherosclerotic plaques (84%), six corresponding subgingival plaque samples were reported as positive. All four positive atherosclerotic plaque samples were also positive for bacterial DNA in the subgingival plaque samples (Table 1).
| Variable | Atherosclerosis | Total | |
|---|---|---|---|
| Negative | Positive | ||
| Subgingival | |||
| Negative | 15 | 0 | 15 |
| Positive | 6 | 4 | 10 |
| Total | 21 | 4 | 25 |
Cross Table of the Frequency of Porphyromonas gingivalis Bacteria in Atherosclerotic and Subgingival Samples a
In 15 out of the 25 examined samples (60%), bacterial DNA was not detected in either the subgingival or atherosclerotic plaques. Conversely, bacterial DNA was detected in both the subgingival and atherosclerotic plaques in 4 cases. Thus, there was an agreement between the two groups in nineteen cases. The McNemar test was employed to compare the two groups (atherosclerotic and subgingival samples) within each patient, and the results revealed statistically significant differences (P-value = 0.03).
Nine of the examined corpses had a medical history of diabetes and cardiovascular diseases. The subgingival and atherosclerotic plaques contained bacterial DNA in two of these cases. Applying the McNemar test in these cases did not reveal a significant difference between the atherosclerotic and subgingival groups (correlation coefficient r = 0.500). Therefore, within each patient with a history of cardiovascular disease and diabetes, there was no significant distinction between the atherosclerotic and subgingival plaque samples.
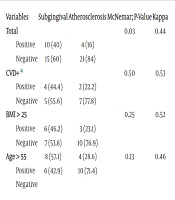
As presented in Table 2, the Spearman correlation coefficient demonstrated a moderate correlation (r = 0.53) between the atherosclerotic and subgingival groups. Table 3 provides an overview of the Kappa agreement coefficient, pairwise comparisons using the McNemar test, and corresponding P-values, assessing the agreement between the atherosclerotic and subgingival groups. The Kappa test revealed poor inter-group agreement (44%). However, a modest agreement was observed between the subgingival and atherosclerotic samples across all subjects. Notably, this agreement coefficient increased among individuals with a history of cardiovascular disease and diabetes, BMI over 25 kg/m2, and age over 55 years. Furthermore, McNemar's test indicated no statistically significant difference between the results of the two atherosclerotic and subgingival plaque samples in high-risk individuals (P-value > 0.05).
| Variables | Atherosclerosis | Total | |
|---|---|---|---|
| Negative | Positive | ||
| Subgingival | |||
| Negative | |||
| Count | 15 (60) | 0 | 15 |
| Expected Count | 12.6 | 2.4 | 15 |
| Positive | |||
| Count | 6 | 4 | 10 |
| Expected Count | 8.4 | 1.6 | 10 |
| Total | |||
| Count | 21 | 4 | 25 |
| Expected Count | 21 | 4 | 25 |
| Measure of Agreement | |||
| Value of Kappa | 0.444 | ||
Correlation Coefficient Between the Results of Two Atherosclerotic and Subgingival Groups
| Variables | Subgingival | Atherosclerosis | McNemar; P-Value | Kappa |
|---|---|---|---|---|
| Total | 0.03 | 0.44 | ||
| Positive | 10 (40) | 4 (16) | ||
| Negative | 15 (60) | 21 (84) | ||
| CVD+ a | 0.50 | 0.53 | ||
| Positive | 4 (44.4) | 2 (22.2) | ||
| Negative | 5 (55.6) | 7 (77.8) | ||
| BMI > 25 | 0.25 | 0.52 | ||
| Positive | 6 (46.2) | 3 (23.1) | ||
| Negative | 7 (53.8) | 10 (76.9) | ||
| Age > 55 | 8 (57.1) | 4 (28.6) | 0.13 | 0.46 |
| Positive | 6 (42.9) | 10 (71.4) | ||
| Negative |
Summary of Kappa Agreement Coefficient, Pairwise Comparison of Two Groups and P-Value for Agreement Between Atherosclerotic and Subgingival Groups
5. Discussion
Reviewing research conducted over the past two decades reveals that certain microorganisms and common risk factors can contribute to the development of atherosclerotic plaques. One such bacterium is P. gingivalis, an anaerobic Gram-negative bacterium known for its role in initiating inflammation and tissue destruction in periodontal disease (13, 18).
Periodontitis, characterized by oral microbiota dysbiosis, is a multifactorial inflammatory disease that destroys periodontal tissues and bone (19). Numerous studies have established a significant association between chronic periodontitis and other conditions like rheumatoid arthritis, diabetes, and cardiovascular disease (20, 21).
The inflammatory atherosclerosis hypothesis posits that atherosclerosis is an inflammatory response triggered by agents that cause damage to the endothelial cells of blood vessel walls (7). In addition to directly infecting cardiac tissue, certain infectious agents can induce and sustain inflammation, indirectly contributing to cardiovascular disease (1, 22). Other risk factors for atherosclerosis include hypertension, hypercholesterolemia, diabetes, obesity, smoking, and physical inactivity (4).
The association between oral health and atherosclerosis was initially suggested by Mattila et al., who found a strong correlation between poor dental hygiene and acute myocardial infarction (23). Mendez et al. identified periodontitis as an independent risk factor for peripheral vascular disease. The presence of periodontopathic bacteria, including P. gingivalis and Streptococcus sanguis, in atherosclerotic lesions was first reported in a study investigating the impact of various infections on the development of carotid artery plaques (24).
The oral cavity serves as a significant reservoir of diverse microorganisms that can enter the bloodstream of individuals with periodontitis through activities like chewing and tooth brushing, which occur multiple times a day (25, 26).
Research indicates that the prevalence of P. gingivalis in healthy adults ranges from 16.8% to 25%, highlighting its presence even in individuals without apparent periodontal issues. For instance, a study published in the Journal of Clinical Microbiology reported a detection rate of 25% in healthy subjects, contrasting with a higher prevalence of 79% in individuals with periodontitis. This disparity underscores the potential subclinical presence of P. gingivalis in healthy populations, suggesting a complex relationship between this bacterium and oral health (27).
In the present study, P. gingivalis was detected in 45% of subgingival plaque samples and 16% of atherosclerotic plaque samples. A pairwise comparison of atherosclerotic and subgingival samples within each patient revealed a statistically significant difference in the results. Six of the 21 atherosclerotic samples negative for P. gingivalis had positive subgingival samples. Furthermore, all four positive atherosclerotic plaque samples also tested positive in examining subgingival plaques. The correlation between the atherosclerotic and subgingival groups was modest (correlation coefficient r = 0.5), and the agreement between the atherosclerotic and subgingival groups, as assessed by the Kappa test, was 44%. Atarbashi-Moghadam et al. reported the presence of P. gingivalis in 43.47% of subgingival and 13.04% of atherosclerotic plaque samples, which was statistically significant. This study is the same as the current study regarding sample size, the microbiological investigation method, the statistical test, and the results. It confirms the relationship between P. gingivalis in subgingival and atherosclerotic plaques (1).
In the study by Altayeb et al., the load of periodontal bacteria in atherosclerotic plaque samples was much higher in patients with periodontitis compared to the healthy group, and pathogens play a role in periodontitis, especially P. gingivalis and Tannerella forsythia, were also found in atherosclerotic plaques. This study showed that the presence of these bacteria in atherosclerotic plaques is attributed to the severity of periodontitis and bacterial load of subgingival plaques. The presence of periodontal pathogens in atherosclerotic plaques showed a relationship between periodontitis and cardiovascular diseases, which is consistent with the results of the present study (3).
Gaetti-Jardim et al. conducted a study that aligns with the present research, as they found no significant association between the presence of periopathogenic bacteria in atherosclerotic plaques and factors such as age, sex, number of teeth, or smoking. P. gingivalis was the most frequently detected bacterium (53.8%) in atherosclerotic plaque samples from patients with periodontitis. The authors of that study suggested that the presence of periopathogenic bacteria in atherosclerotic plaques of individuals with periodontitis indicates a non-random occurrence and implies their potential involvement in cardiovascular diseases, consistent with the current study's findings (4).
In another study by Marcelino et al., they reported the presence of P. gingivalis in 75% of subgingival plaque samples and 50% of atherosclerotic plaque samples, corroborating the present study and confirming the relationship between P. gingivalis in subgingival and atherosclerotic plaques (28).
Szulc et al. also found the presence of P. gingivalis in 74% of subgingival plaque samples and 23% of atherosclerotic plaque samples. Their findings demonstrated the frequent occurrence of P. gingivalis in atherosclerotic plaques of patients with periodontitis, which is consistent with the current study (7).
On the other hand, Cairo et al. reported the presence of P. gingivalis in 53% of subgingival plaque samples (15). However, they did not detect it in any of the atherosclerotic plaque samples. They did not observe a relationship between the presence of periodontal bacteria in subgingival plaques and the development of atherosclerotic plaques, which contradicts the results of the present study. This discrepancy could be attributed to the smaller sample size in their study, which may have hindered the detection of a relationship between the presence of P. gingivalis in subgingival plaques and atherosclerotic plaques. Furthermore, the methods employed in the two studies differ, as Cairo et al. collected samples from the carotid arteries of living individuals. In contrast, our study collected samples from the coronary arteries of deceased individuals (15). Aimetti et al. reported the presence of P. gingivalis in 63% of subgingival plaque samples and did not report the presence of this bacterium in any of the atherosclerotic plaque samples. They found no relationship between P. gingivalis in subgingival and atherosclerotic plaques. They took their sample from carotid artery plaques. The discrepancy between the results can be attributed to the different immune responses of the host, the different study populations, and the different sample collection techniques (11).
Additional analyses conducted in our study revealed that when considering only the high-risk group of individuals with a history of cardiovascular disease and diabetes, there was no statistically significant difference between the atherosclerotic and subgingival plaque samples. However, the small size of our study may have influenced these findings. Given that advanced age, high BMI, and underlying systemic conditions such as diabetes and smoking are significant risk factors for cardiovascular diseases, it is imperative to prioritize these factors and conduct further investigations in future studies.
One limitation of our research was the difficulty in obtaining suitable samples. Rigor mortis posed challenges in opening the mouth, thereby hindering access to posterior teeth during subgingival plaque sampling. Additionally, the small sample size and focus solely on the DNA of P. gingivalis bacteria were areas that could be improved in our study. Future studies can explore the examination of other periopathogenic bacteria in similar contexts. Furthermore, our study only examined the existence of an agreement between samples without investigating cause-and-effect relationships. It is worth considering that by focusing on larger sample sizes and minimizing data dispersion in terms of age, sex, and systemic conditions, a stronger agreement between subgingival and atherosclerotic samples can potentially be achieved, particularly within high-risk groups.
Future studies could use next-generation sequencing to fully characterize all oral and atherosclerotic plaque specimens to better understand the role of periopathogenic bacteria in atherosclerotic plaque development and progression. This approach would provide valuable insights into the intricate mechanisms underlying the involvement of periopathogenic bacteria in atherosclerosis.
5.1. Conclusions
In conclusion, despite the limitations of our study, the results highlight a significant relationship between the presence of P. gingivalis in subgingival and atherosclerotic plaques in coronary arteries. Patients with chronic periodontitis exhibited positive detection of this pathogen in their atherosclerotic plaques, indicating a potential link between periodontal disease and cardiovascular health. These findings underscore the importance of emphasizing effective periodontal therapy as a potential intervention for improving cardiovascular outcomes in individuals with cardiovascular disease. Further research with larger sample sizes and comprehensive analysis of other periopathogenic bacteria is warranted to better understand this association and establish causality.