1. Background
In December 2019, the coronavirus disease 19 (COVID-19) pandemic resulted in a pneumonia outbreak caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan City, China. The disease affected millions of people worldwide (1), leading to more than 673 million contractions and 3 million deaths worldwide as of January 1, 2023 (2). COVID-19 is a highly infectious disease that affects the lungs and immune system and emerges clinically with fever, cough, and shortness of breath (3, 4). The clinical course of the SARS-CoV-2 infection varies considerably, with some patients showing a mild disease while others develop more severe conditions that may require a long time to recover or even be fatal (3, 5). COVID-19 is a highly pathogenic disease associated with deregulated immune responses (5, 6). Despite the worldwide efforts directed at the study of COVID-19, its underlying molecular and pathophysiological mechanisms have largely remained puzzling (7). As a result, new approaches are required to manage COVID-19 and improve the prognosis of the disease (8).
Numerous molecules have been evaluated as early diagnostic markers of COVID-19, but there are yet no universally accepted diagnostic biomarkers for this disease (9). Calprotectin (S100A8/A9, Calgranolin A, B, Alarmins, and CLP) is a small heterodimer protein binding to calcium and zinc. Calprotectin is primarily produced by and released from macrophages and neutrophils, with a serum level between 0.1 and 1.6 µg/mL (10, 11). This cytosolic heterodimer protein often plays an important role in regulating immune responses by stimulating TLR4-mediated inflammatory pathways. Furthermore, its predictive role in the outcome of rheumatoid arthritis and inflammatory bowel disease has been reported (10, 11). Calprotectin can also act as an antibacterial, antifungal, antiproliferative, and immunomodulating agent (12). Therefore, during inflammation, calprotectin facilitates the maintenance of homeostasis, but when it is expressed and secreted in excess amounts, it disturbs the balance of inflammatory processes (13). As an acute phase reactant, calprotectin expression often increases following infections, traumas, and inflammatory diseases when neutrophils and other cells are stimulated to release cytokines, including calprotectin. The stability of calprotectin at room temperature has made it a potential biomarker for monitoring inflammatory conditions such as COVID-19 (10, 14).
Neutrophil-derived heparin-binding protein (HBP, known as azurocidin or CAP-37) is also a key player during bacterial infections. The elevation of HBP has been detected before the onset of limb dysfunction in patients with severe COVID-19; therefore, HBP can be used as a prognostic marker in COVID-19 (15). This protein is stored in the secretory vesicles and azurophilic granules of neutrophils and is released upon the activation of these immune cells (16). Since pre-formed and pre-packed HBP is ready to be released upon the onset of inflammation, this protein is considered a promising biomarker in infections. Plasma HBP levels rise 12 hours before the onset of symptoms of circulatory failure and limb dysfunction in ill patients (17). Studies have shown that neutrophil activation is an essential phase in the pathophysiology of COVID-19 and may be a main contributor to severe SARS-CoV-2 infection and bacterial sepsis (18). Bacterial infections are relatively rare in COVID-19, observed in less than 10% of infected patients, meaning that elevated levels of HBP in this condition cannot be merely explained by bacterial infections, but it is plausible to attribute this phenomenon to the viral infection (15).
Nowadays, distinguishing severe from non-severe COVID-19 is an interesting topic of research, and studies have suggested several biomarkers, including C-reactive Protein (CRP), lactate dehydrogenase (LDH) activity, ferritin (FERR), and neutrophil (NEU) and platelet (PLT) counts (19), for this purpose. Little is currently known about how neutrophil activation can be connected to COVID-19 severity and inflammatory status. Respiratory infections require early detection and adequate treatment. Early interventions targeting HBP and calprotectin may improve the prognosis of patients with severe COVID-19. Mounting evidence on pathophysiological pathways and inflammatory factors in patients with COVID-19 suggests that immunomodulatory factors may mitigate the severity of the disease (20).
2. Objectives
Collectively, it is hypothesized that serum inflammatory factors, calprotectin, and HBP can be possible diagnostic and prognostic markers for COVID-19. Therefore, in this study, we aimed to determine the serum levels of calprotectin and HBP in patients with COVID-19 and assess their association with some other serological indicators, including LDH activity, platelet count (PLT), neutrophil count (NEU), ferritin (FERR), and CRP. Our findings can provide crucial information about the potential applicability of calprotectin as a prognostic or diagnostic biomarker in COVID-19. The potential association of serum calprotectin level with biomarkers such as CRP, NEU, and LDH activity may help physicians predict the severity of COVID-19.
3. Methods
3.1. Sample Collection
A total of 70 pharyngeal swab samples were obtained from 38 men and 32 women with an average age of 45.87 ± 10.98 years, including 35 outpatients with COVID-19 admitted to the Ayatollah Kashani Hospital (Tehran) and 35 healthy controls. In this case-control study, pharyngeal swab samples were collected from all participants from August 5 to September 30, 2022. Then, SARS-CoV-2 testing was conducted by RT-qPCR technique using the 2019-nCoV2 Nucleic Acid Diagnostic Kit (Sansure Biotech, Changsha-China) to confirm (in patients) or exclude (in controls) the infection. The subjects underwent clinical examinations by experts, and the final diagnosis of COVID-19 was confirmed based on clinical and paraclinical (RT-qPCR testing) findings. People with a history of multiple organ failure, as well as people with cardiovascular diseases, a history of organ transplantation, diabetes, and inflammatory diseases, were excluded from the study.
The sample size was estimated using the formula N = (Z1-α/2 + Z1-β)2 × (δ12+ δ22) / (µ1 - µ2)2 and considering the statistical power of 90% (Z1-β), the confidence level of 95% (Z1-α/2), and possible withdrawals, 35 individuals were included in each study group. Blood samples (10 mL) were collected from all participants into anticoagulant-free tubes. The serum levels of HBP, calprotectin, FERR, and CRP, as well as PLT and NEU counts and LDH activity, were determined.
Formal written informed consent was obtained from all participants, and the study received ethical approval from the Hamedan University of Medical Sciences (IR.UMSHA.REC.1401.288).
3.2. Determination of Serum Calprotectin Concentration
Creative Diagnostics Human Calprotectin ELISA kit (Creative Diagnostics, USA) was used to determine serum calprotectin level. Briefly, samples were transferred into a microplate pre-coated with polyclonal anti-human S100A9 antibodies (Creative Diagnostics, USA). After 60 minutes of incubation and washing, horseradish peroxidase-labeled anti-human S100A8 polyclonal antibodies (Creative Diagnostics, USA) were added, and the samples were incubated for another 60 min. Following the washing step, the residual conjugate was reacted with a tetramethylbenzidine substrate solution. The reaction was stopped by adding an acidic solution, and the absorbance of the resulting yellow product was measured, which was proportional to the concentration of S100A8/A9. A standard curve was drawn by plotting the 450 nm absorbance values, and calprotectin concentration in unknown samples was determined using the standard curve.
3.3. Determination of Serum HBP Level
The level of HBP in serum samples was determined using the MyBioSource Sandwich ELISA Immunoassay kit (MyBioSource Inc., San Diego-USA) according to the manufacturer’s instructions. Briefly, serum samples were poured into the wells on microplates pre-coated with anti-HBP primary antibodies (MyBioSource, USA). After incubation of the microplates at room temperature for 2 h, conjugated secondary antibodies (Creative Diagnostics, USA) were added to the samples to bind the protein of interest. Next, tetramethylbenzidine was added to each well, and the reaction was stopped by adding the H2SO4 solution (2M). Finally, the optical density was recorded at 450 nm, and the HBP serum level was quantified and expressed as pg/mL.
3.4. Measurement of Serum CRP Level
Serum CRP level was quantified using a CRP kit (Pars Azmun, Iran) based on determining the turbidity resulting from the formation of a complex between CRP present in the patient’s sample and sensitized antibodies against human CRP. The degree of turbidity detected using an immunoturbidimetry method, was directly related to the concentration of CRP in the sample, which was reported as mg/dL.
3.5. Determination of PLT and NEU Counts
Neutrophils and platelets were enumerated by a Sysmex cell counter (Sysmex KX-21-Japan) and expressed as a percentage (%) of white blood cells (for NEU) and the number of cells per microliter of blood (for PLT).
3.6. Determination of Serum Ferritin Concentration
Serum ferritin level (FERR) was measured using the Dia. Pro ELISA diagnostic kit (Dia. Pro Diagnostic Bioprobes, Milan-Italy) based on a quantitative immunoenzymometry method. The assay used specific monoclonal antibodies and employed the sandwich ELISA method. Anti-ferritin monoclonal antibodies (Dia. Pro Diagnostic Bioprobes, Milan-Italy) were used to conjugate with the HRP enzyme as spacers between the solid phase and anti-ferritin antibodies. By adding the serum sample, ferritin molecules reacted with the antibodies and formed sandwiches between conjugated antibodies and the antibodies bound on the solid phase. The rate of complex formation was measured by reading optical density at 450 nm. The results were reported as ng/mL.
3.7. Measurement of LDH Enzymatic Activity
To determine LDH activity, an LDH kit (Pars Azmoun, Iran) was used, in which the LDH enzyme present in the sample would convert lactic acid to pyruvic acid in a reversible reaction. In this reaction, "NAD+" acts as a hydrogen acceptor and turns into "NADH." The difference between the optical absorption of NADH and NAD+ was the basis for determining LDH enzymatic activity as U/L.
3.8. Statistical Methods
All statistical analyses were performed using SPSS software version 26.0 (IBM, USA). Descriptive statistics included mean ± SD, median, and interquartile range (IQR). The normality of data distribution was checked by the Shapiro-Wilk test. Comparison of normally distributed variables between the two groups was conducted using the student-t test, while the Mann–Whitney U test was used to compare variables with non-normal distribution. Logistic regression analysis was used to assess correlations between variables. Potential COVID-19 predictors were entered into a multivariable logistic regression model with the stepwise backward logistic regression mode. The correlation of HBP and calprotectin with other biomarkers was assessed using the Spearman correlation test, and a P-value < 0.05 was considered statistically significant.
4. Results
4.1. Characteristics of Patients
The analysis of pharyngeal swab specimens from all participants (38 men and 32 women) confirmed the presence of SARS-CoV-2 viral RNAs only in the patients (n = 35). There was no significant difference in the mean age of the patients (45.40 ± 10.34 years) and healthy control subjects (46.34 ± 11.71 years).
4.2. Serum Inflammatory Biomarkers in the Two Groups
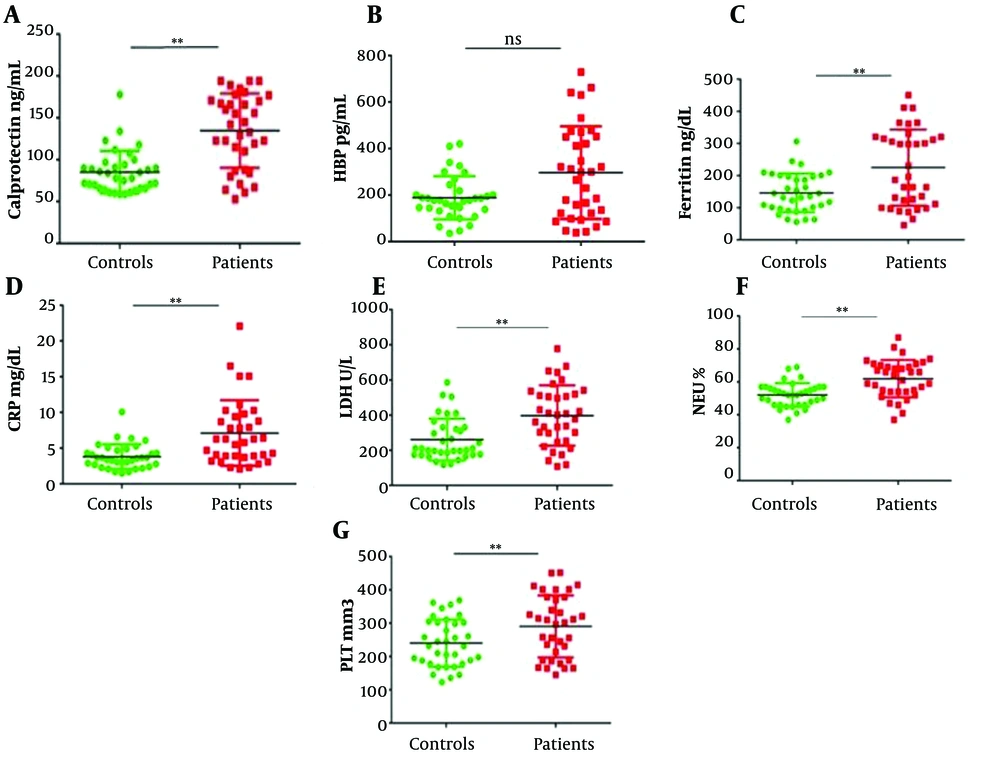
COVID-19 patients had significantly higher levels of serum calprotectin compared with control subjects (P < 0.05, Figure 1A), while the elevation of HBP levels in the sera of COVID-19 patients did not reach a statistically significant difference compared to controls (P > 0.05, Figure 1B). Serum FERR and CRP levels were also significantly higher in COVID-19 patients compared to healthy subjects (P < 0.05, Figure 1C and D, respectively). Also, COVID-19 patients had markedly higher LDH activity (P < 0.05, Figure 1E), NEU count (Figure 1F), and PLT count (Figure 1G) compared to controls.
Comparison of inflammatory markers between healthy individuals and patients with COVID-19 (A-G). All factors, except for HBP, were significantly higher in the case group (**P < 0.05). CRP, C-reactive protein; FERR, ferritin; HBP, heparin-binding protein; LDH, lactate dehydrogenase; NEU, neutrophil; PLT, platelets.
4.3. Clinical Relevance of Calprotectin and Other Inflammatory Factors in COVID-19 Patients
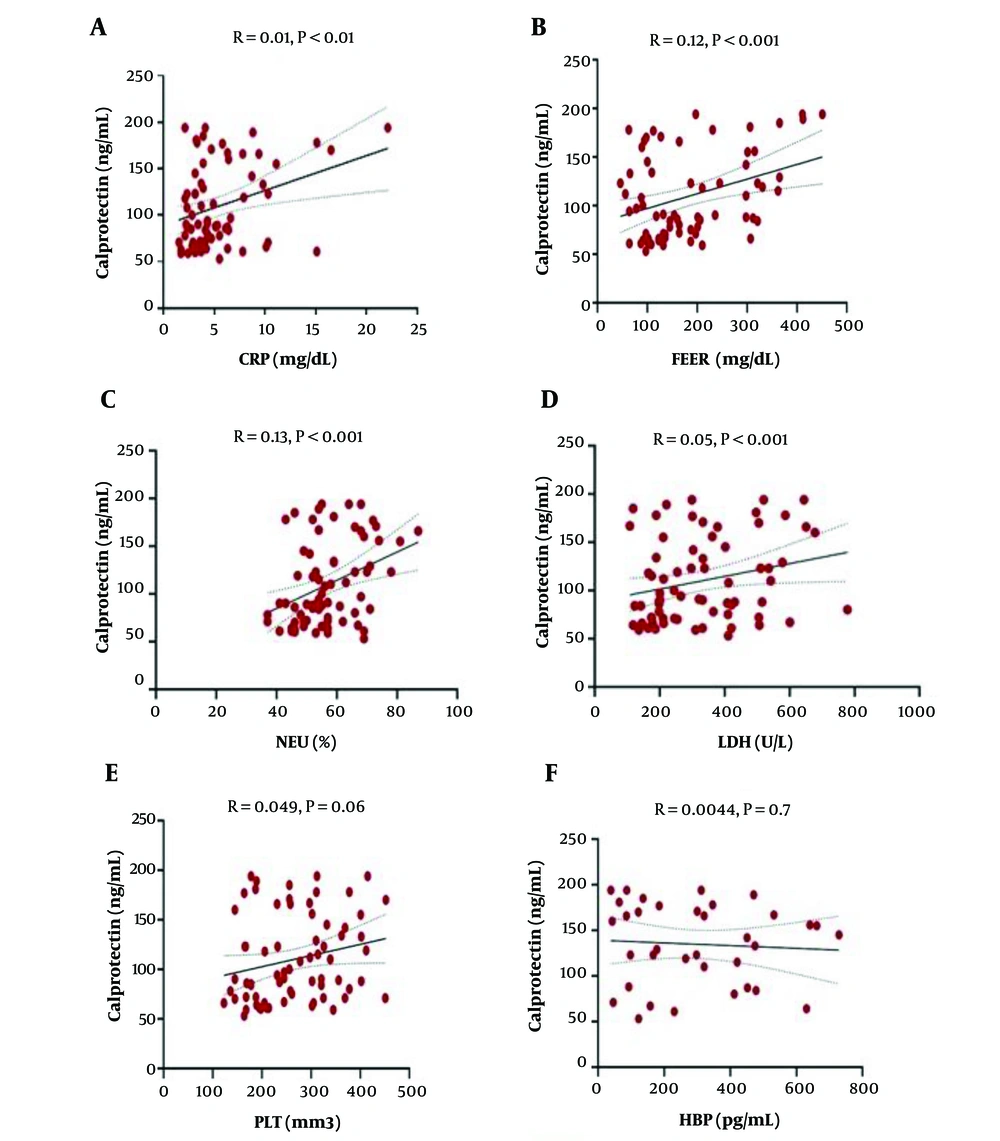
Logistic regression analysis was performed to determine possible correlations between serum calprotectin and other inflammatory markers. Statistical analysis showed a significant association between serum calprotectin and serum levels of CRP and FERR, NEU%, and LDH activity (P < 0.001, Figure 2A to Figure 2D, respectively). However, serum calprotectin showed no significant correlation with PLT count (P > 0.001, Figure 2E) and serum HBP level (Figure 2F). In addition, serum HBP level showed no correlation with serum CRP and FERR levels, LDH activity, NEU%, and PLT count (Table 1).
The correlation of calprotectin level with other inflammatory factors in the two study groups. Correlation with CRP (A), FERR (B), NEU (C), and LDH (D) as determined by logistic regression analysis. There was no significant relationship between calprotectin and PLT (E) and HBP (F). Other indicators that did not exhibit considerable diagnostic or prognostic values were not shown.
| B | S.E | P-Value | OR (95% CI) | |
|---|---|---|---|---|
| Calprotectin × PLT | 0.000019 | 0.000005 | 0.002 | 1.000019 (1.000007-1.000034) |
| HBP× PLT | 0.000017 | 0.000006 | 0.005 | 1.000017 (1.000005-1.000028) |
| Calprotectin × LDH | 0.000068 | 0.000018 | <0.001 | 1.000068 (1.000032-1.00010) |
| HBP× LDH | 0.00002 | 0.000007 | 0.003 | 1.00002 (1.000007-1.000033) |
| Calprotectin × HBP | 0.000068 | 0.000021 | 0.001 | 1.000068 (1.000027-1.00011) |
| Calprotectin × Neu | 0.0006 | 0.00015 | <0.001 | 1.0006 (1.00034- 1.0009) |
| HBP× Neu | 0.00010 | 0.00034 | 0.002 | 1.00010 (1.000037-0.00017) |
| Calprotectin × CRP | 0.0057 | 0.002 | <0.001 | 1.0057 (1.0025-1.0089) |
| HBP × CRP | 0.0014 | 0.00043 | 0.001 | 1.0014 (1.00052-0.0022) |
| Calprotectin × FERR | 0.00012 | 0.000034 | <0.001 | 1.00012 (1.000054-1.00019) |
| HBP × FERR | 0.000031 | 0.000011 | 0.004 | 1.000031 (1.000010-1.000052) |
4.4. Predictive Sensitivity and Specificity of Inflammatory Markers for COVID-19
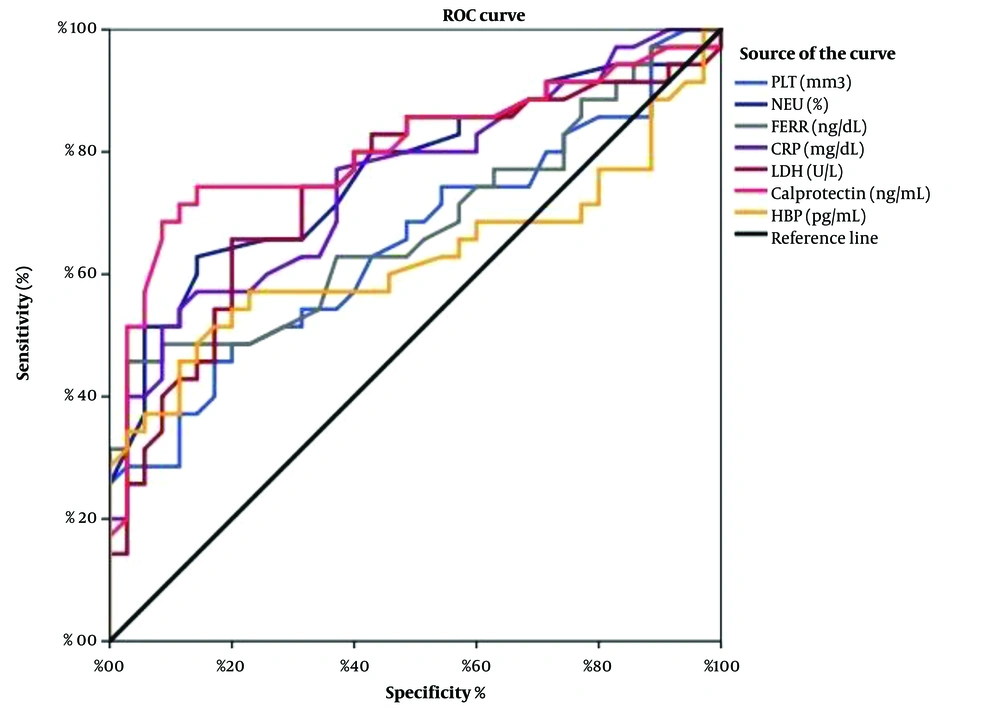
Receiver operating characteristic (ROC) curves were used to predict the sensitivity and specificity of the inflammatory markers investigated. Areas under the curve (AUCs) and P-values were obtained as AUC = 0.889 (P < 0.001) for calprotectin, AUC = 0.627 (P = 0.68) for HBP, AUC = 0.752 (P < 0.001) for CRP, AUC = 0.678 (P < 0.001) for ferritin, AUC = 0.651 (P < 0.001) for PLT count, AUC = 0.766 (P < 0.001) for NEU count, and AUC= 0.742 (P < 0.001) for LDH activity (Figure 3). Serum calprotectin level demonstrated the highest sensitivity and specificity compared with other variables.
5. Discussion
The SARS-CoV-2 virus has been infecting people around the world for almost the last four years. It is considered the main cause of COVID-19 disease, which is an infection with mild to significant clinical manifestations (21, 22). COVID-19 is characterized by upper respiratory tract infection in the majority of patients, leading to respiratory failure in patients with intense acute lung injury (23), which is followed by cytokine storm, multiorgan failure, and death (24). Therefore, due to the expansion of inflammatory responses, it is believed that monitoring the levels of inflammatory biomarkers during the course of the disease may provide comprehensive knowledge of the severity of COVID-19 or the outcomes of patients (25). Thus, in the present study, serum levels of calprotectin, HBP, ferritin, and CRP, as well as LDH activity and NEU and PLT counts, were determined in COVID-19 patients and compared with similar markers in healthy subjects. A significantly higher level of calprotectin, ferritin, CRP, LDH activity, and NEU and PLT counts were observed in COVID-19 patients compared with controls. In addition, serum calprotectin was observed to be significantly associated with serum FERR and CRP levels, LDH activity, and NEU count in COVID-19 patients.
Calprotectin is a serum protein secreted especially by neutrophils in response to infections. In patients with COVID-19, the level of serum calprotectin has been proposed to be associated with deteriorating clinical conditions and reduced survival in patients with pulmonary involvement (26). Recent studies have suggested that calprotectin can be a potentially valuable biomarker to predict clinical course in patients with COVID-19. Accordingly, in the present study, we found elevated levels of calprotectin in the sera of COVID-19 patients compared to healthy subjects. Considering the mounting evidence suggesting serum calprotectin as a risk factor for COVID-19 severity, it is believed that this protein can probably be considered a novel factor for risk stratification, along with CRP, D-dimer, IL-6, and ferritin, in these patients (26). Serum calprotectin was observed to have a positive correlation with the circulatory levels of CRP and ferritin, LDH activity, and neutrophil count. Our results revealed that elevated calprotectin levels, as a marker of neutrophil activation, correlated with the elevation of other inflammatory markers in COVID-19 patients. Very recently, Malik et al. and Henry et al. evaluated the levels of calprotectin in patients with severe and non-severe SARS-CoV-2 infection (27, 28) and witnessed markedly higher levels of calprotectin in patients with severe COVID-19, suggesting this marker as a potentially important prognostic element. These researchers also noted that several biomarkers of lymphopenia, including CRP, procalcitonin, D-dimer, creatine kinase, aspartate aminotransferase, alanine aminotransferase, creatinine, and serum amyloid A, were markedly associated with a poor prognosis in COVID-19 patients (27, 28). Evidence suggests that calprotectin is a reliable early biomarker for predicting the severity of COVID-19 in hospitalized patients (7, 29). Udeh et al. examined the role of calprotectin in predicting severe respiratory failure in intensive care unit (ICU)-hospitalized COVID-19 patients and as a direct predictor of disease severity (9) and the need for ICU admission (30). In another study, Havelka et al. examined the applicability of calprotectin as a diagnostic marker for bacterial infections and a marker for distinguishing between bacterial, mycoplasma, and viral respiratory infections. The results showed that calprotectin was a more useful marker than procalcitonin and HBP in differentiating between bacterial and viral infections, including mycoplasma (31). Calprotectin has recently been reported as a promising serological biomarker for risk assessment in COVID-19 patients (26).
Severe COVID-19 is characterized by neutrophil activation (32) and hyperinflammatory host immune responses, potentially leading to endotheliitis (33), severe respiratory failure, and death (34). Thus, the exaggerated activation of neutrophils in COVID-19 is probably due to unlimited viral replication, tissue hypoxia, and acute inflammation (35). A new inflammatory factor, HBP, has been suggested to be associated with disease progression in patients with severe COVID-19, indicating significantly elevated HBP levels in COVID-19 patients following the exacerbation of its clinical course. It has been reported that serum HBP remains elevated for five days prior to clinical presentation, and higher serum HBP levels are closely associated with worsening pulmonary ventilation (8). Studies on sepsis have revealed that HBP is a more sensitive and specific predictor of clinical outcome than CRP or procalcitonin in patients with severe infections (36, 37). Therefore, we evaluated if elevated HBP and calprotectin levels might play a role in the pathophysiology and progression of severe SARS-CoV-2 infection. It has been shown that HBP is closely associated with the severity of COVID-19, respiratory failure, the levels of other inflammatory factors, coagulation abnormalities, and lung damage (8, 15). Mellhammar et al. examined the possible role of HBP in the pathophysiology of COVID-19 and if this marker could be used to predict the severity of the disease. The results showed that HBP was elevated before the onset of organ dysfunction and cytokine storm in patients (15), suggesting HBP as a prognostic marker in COVID-19 (15). We observed that HBP levels were elevated in patients with COVID-19 in parallel with neutrophil activation, suggesting an association between these events. Therefore, HBP, as an inflammatory factor associated with coagulopathies, seems to be associated with the severity of COVID-19. Xue et al. reported that elevated HBP levels can be associated with prolonged activated thromboplastin and clotting times and increased D-dimer levels (8).
In our study, we found that calprotectin was a better prognostic and diagnostic biomarker for COVID-19 compared to HBP. However, HBP increases before the onset of symptoms and disease presentation. More clinical investigations are needed to determine how HBP is related to COVID-19 and to confirm the role of calprotectin as a diagnostic marker in COVID-19. In addition, it is noteworthy that cytokine storm is present in many patients with severe COVID-19, and the activation of neutrophils can further deteriorate this condition. Regarding the association between calprotectin and neutrophil activation, unlike in other known viral infections, calprotectin, along with ferritin, CRP, LDH activity, and neutrophil count, may be an essential biomarker in COVID-19 and a superb circulatory indicator for its early diagnosis. In the present study, significantly high levels of calprotectin were observed in COVID-19 patients, showing a significant correlation with ferritin, CRP, LDH activity, and NEU. However, people with a history of multiple organ failure, cardiovascular diseases, organ transplantation, diabetes, and inflammatory diseases were excluded from this study.
Our results should be interpreted considering the following notions: (1) The possible effects of ethnicity and gender on biochemical parameters; (2) the effects of infection with different strains of the SARS-CoV-2 virus; and (3) our small sample size. Therefore, more studies are needed to investigate the relationship between the serum level of calprotectin and the severity of COVID-19 and to evaluate its usefulness as a biomarker for monitoring the treatment process.
5.1. Conclusions
According to our results on the relationship between inflammatory factors and COVID-19, calprotectin can be considered a useful serum biomarker for the diagnosis of this disease and a helpful surrogate for monitoring and/or preventing disease progression.