1. Background
Coronavirus disease 2019 (COVID-19) has been a global pandemic since March 2020, as declared by the World Health Organization (WHO), with its initial cases reported in Wuhan, China. Unlike other coronaviruses that primarily affect the respiratory system, SARS-CoV-2 is characterized by respiratory infection, extrapulmonary complications, and multisystem failure (1). Pneumothorax (PTX), pneumomediastinum (PM), and subcutaneous emphysema (SE) represent rare but significant complications in patients with severe COVID-19. These complications may arise from the disease itself or its management (1). Research indicates that these spontaneous complications occur in less than 1% of patients, with approximately 80% resulting from barotrauma due to positive pressure ventilation (PPV) in those with COVID-19 (2). The proposed mechanisms for these complications include increased airway and alveolar pressure leading to alveolar rupture in mechanically ventilated patients. However, in the context of COVID-19 infection, the disease's nature and its immunological and inflammatory responses may also play a crucial role in these complications (2-4). Severe concomitant parenchymal damage, alongside PTX, PM, or SE, has been associated with a high mortality rate in COVID-19 pneumonia (5, 6), underscoring the need for further research into predisposing factors and management strategies for these complications. To the best of our knowledge, no study has yet explored the potential factors that predispose patients to PTX, PM, or SE.
2. Objectives
In this study, we aim to (A) compare the risk factors, characteristics, and outcomes of COVID-19 pneumonia in patients with PTX, PM, or SE (the complicated group) against those without these complications (the uncomplicated group); and (B) present and compare data within the group of patients with complications.
3. Methods
3.1. Study Setting
This case-control study was conducted from March 2020 to September 2021 at Al-Zahra University-affiliated Hospital in Isfahan, Iran. Written consent was obtained from all participants or their guardians. The study protocol received approval from the Ethical Committee at Isfahan University of Medical Sciences (Code of Medical Ethics: IR.MUI.MED.REC.1400.566). The study was prepared and reported in accordance with the STROBE guidelines.
3.2. Sample Size
Our observations indicated that the rate of pulmonary complications among hospitalized patients with COVID-19 pneumonia was 11%. Considering an alpha of 0.05, a beta of 0.2, and accounting for an anticipated 4% rate of missed cases, it was estimated that a total of 162 patients would be sufficient for the study.
3.3. Identification of Cases and Control Subjects
Cases, or Complicated Patients (CP), were identified based on a positive PCR test for COVID-19 and reported from lung high-resolution computed tomography (HRCT). These patients also had the International Classification of Diseases, tenth revision (ICD-10) codes for PTX, PM, or SE recorded in their medical records during hospital admission, based on their clinical course, lung HRCT, or chest x-ray findings. Patients with incomplete medical records were excluded.
For each case, one control subject was selected from our hospital database. Control subjects were COVID-19 patients matched by age, gender, and day of admission to our center. Control subjects with pulmonary complications (i.e., PTX, PM, or SE) or incomplete medical records were excluded. The matching process utilized the standardized mean difference.
3.4. Collection of Variables
We collected demographic data, medical history, lists of medications, laboratory results, surgical reports on chest tube insertion, anesthesia reports on intubation and ventilator settings, lung HRCT and PCR results, and the final status of patients from the database or by contacting them via telephone.
3.5. Statistical Analysis
Data analysis was conducted using IBM SPSS Statistics for Windows (version 26.0). Fisher's exact test and the chi-square test were employed to analyze qualitative variables. The Shapiro-Wilk test, along with Q-Q plots, was utilized to assess the distribution of numerical variables. Parametric data were analyzed using the independent samples t-test and are presented as mean ± standard deviation. Non-parametric data were analyzed using the Mann-Whitney U test. The logistic regression model was applied to evaluate the impact of significant demographic (age, gender, BMI), medical history, and laboratory factors on the study's desired outcomes (pulmonary complications and mortality).
4. Results
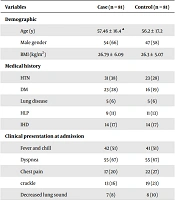
The study included 81 pairs of patients with COVID-19 pneumonia. Among the complications studied, PTX was the most common, followed by EM and PM (76%, 38%, and 28%, respectively). The demographic data, including age, gender, BMI, history of hypertension (HTN), type 2 diabetes mellitus (DM), hyperlipidemia (HLP), lung diseases (including smoking lung, asthma, and COPD), ischemic heart disease (IHD), and positive clinical presentation at admission, are presented in Table 1; no significant differences were observed between the two groups. Additionally, when the data were analyzed based on the type of pulmonary complication within the case group, no significant differences were found in demographic or medical history.
During their hospital stay, 70% of patients in each group were intubated. Table 2 compares ventilator settings, including mean tidal volume (TV), peak inspiratory pressure (PIP), positive end-expiratory pressure (PEEP), fraction of inspired oxygen (FiO2), and respiratory rate (RR), none of which showed statistically significant differences between the complicated patients (CP) and uncomplicated patients (UCP) groups.
| Variables | Case (n = 81) | Control (n = 81) |
|---|---|---|
| Demographic | ||
| Age (y) | 57.46 ± 16.4 a | 56.2 ± 17.2 |
| Male gender | 54 (66) | 47 (58) |
| BMI (kg/m2) | 26.79 ± 6.09 | 26.3 ± 5.07 |
| Medical history | ||
| HTN | 31 (38) | 23 (28) |
| DM | 23 (28) | 16 (19) |
| Lung disease | 5 (6) | 5 (6) |
| HLP | 9 (11) | 11 (13) |
| IHD | 14 (17) | 14 (17) |
| Clinical presentation at admission | ||
| Fever and chill | 42 (51) | 41 (51) |
| Dyspnea | 55 (67) | 55 (67) |
| Chest pain | 17 (20) | 22 (27) |
| crackle | 13 (16) | 19 (23) |
| Decreased lung sound | 7 (8) | 8 (10) |
Abbreviations: IHD, ischemic heart disease; DM, diabetes mellitus; HLP, hyperlipidemia; HTN, hypertension; BMI, body mass index.
aThe numerical variables are presented as mean ± standard deviation and nominal variables are presented as No. (%).
| Setting | Case (n = 55) | Control (n = 55) |
|---|---|---|
| TV (mL) | 469.5 ± 71 a | 476.4 ± 74 |
| RR, rate/min | 17.6 ± 4.3 | 18 ± 4.5 |
| PIP, cmH2O | 15.6 ± 4.2 | 15.8 ± 4 |
| PEEP, cmH2O | 9.8 ± 12.5 | 8.4 ± 1.8 |
| FiO2 | 99.5 ± 57.1 | 108.7 ± 77.6 |
Abbreviations: TV, tidal volume; RR, respiratory rate; PIP, peak inspiratory pressure; PEEP, positive end-expiratory pressure; FiO2, fraction of expired oxygen.
a Values are expressed as mean ± SD.
Specific laboratory data were significantly higher in patients with complicated COVID-19 pneumonia (Table 3). Logistic regression analysis of studied variables as potential risk factors for COVID-19 pneumonia complications, with the complication as the dependent variable, revealed that only lactate dehydrogenase (LDH) in units per liter (u/L) (B = -0.002 and Exp (B) = 0.998 with a 95% confidence interval (CI) of 0.997 - 0.999) and Troponin in nanoliters per milliliter (nL/mL) (B = -0.466 and Exp (B) = 0.629 with a 95% CI of 0.51 - 0.77) were significantly associated. In the case group, after categorizing based on the type of complication, LDH levels were significantly higher in the PTX group compared to patients without PTX (1031.74 ± 436.65 vs. 858.42 ± 211.30, P = 0.02).
| Variables | Case (n = 81) | Control (n = 81) | P-Value a |
|---|---|---|---|
| WBC count (/µL) | 9933.3 ± 5367 | 7751 ± 3231.6 | > 0.001 |
| Lymphocyte count (/µL of blood) | 455 ± 454.9 | 14.4 ± 7.1 | < 0.001 |
| Lymphopenia; No. (%) | 59 (72) | 44 (54) | 0.01 |
| CRP (µg/mL) | 70.9 ± 35.2 | 60.7 ± 21.3 | 0.02 |
| ESR | 44.6 ± 24.2 | 44 ± 66.7 | 0.9 |
| D-dimer | 1754.1 ± 1400 | 1294 ± 1197 | 0.02 |
| LDH (u/L) | 991 ± 401 | 750 ± 298.9 | < 0.001 |
| Troponin (nL/mL) | 10.2 ± 11 | 3.6 ± 1.8 | < 0.001 |
| PH | 7.3 | 7.3 | 0.1 |
| PCO2 | 39 ± 10.4 | 39 ± 10.3 | 0.9 |
| HCO3 | 21.9 ± 5.4 | 21.9 ± 5.3 | 0.9 |
a All the P values are not significant with the level > 0.05
There was a significant association between patients' final status and complications; the death rate in the CP group was 70% compared to 14% in the UCP group (P < 0.0001; B = 2.61, Exp(B) = 13.65 with a 95% CI of 6.28-29.69). The mortality rate among different types of complicated patients was 77.4% for PTX (P = 0.02), 65.2% for PM (P = 0.59), and 74.2% for EM (P = 0.55). Logistic regression on the final status across different types of pulmonary complications did not identify any associated factors.
The case group experienced a significantly longer hospital stay. For intubated patients in the CP group, the average length of stay was 43.1 ± 5.2 days, compared to 27.3 ± 8.6 days for those not intubated (P < 0.001). Furthermore, the length of stay was significantly shorter in the UCP group, with intubated patients staying an average of 31.4 ± 7.5 days and non-intubated patients 8.2 ± 3.4 days (P < 0.001).
5. Discussion
The findings of our study revealed that inflammatory markers were higher in complicated patients, except for ESR levels. Moreover, no comorbid disease increased the risk of pulmonary complications, the type of complication, or the mortality rate, although mortality was significantly higher in the complicated group. Furthermore, none of the evaluated risk factors were associated with complications. However, the hospital length of stay was longer for detailed or intubated patients than for the other group.
COVID-19 pneumonia damages the alveoli, and with or without intubation, the damaged single-cell layer may cause pulmonary problems like PTX, PM, or EM. However, due to the progression of pneumonia and difficulty in breathing and oxygenation, known as pulmonary failure, patients require intubation and mechanical ventilation. Indeed, this extra pressure may burst the susceptible alveoli and rupture the normal ones, resulting in the same complications. The mechanism and pathophysiology of air leak syndrome due to ventilation begin with the rupture of an overdistended alveolus, which may be due to generalized air trapping or uneven distribution of gas. The air dissects along the perivascular connective tissue sheath toward the hilum, resulting in a pneumomediastinum or into the pleural space, producing a pneumothorax. The high negative intrathoracic forces created when the neonate starts breathing occasionally disrupt the alveolar epithelium, allowing air to move from the alveoli into extra-alveolar soft tissues or spaces (7). Furthermore, barotrauma caused by mechanical ventilation has been accused of being an essential factor in developing air leaks (8). Long-term mechanical ventilation in COVID-19 patients can lead to cyst ruptures and air escape, potentially causing PTX, PM, or EM (9-11). In our study, various ventilatory parameters were associated with a higher risk of air leak syndrome in patients. Despite barotrauma from mechanical ventilation being the primary cause of air escape and air leak syndrome, not every case of air leak syndrome in our study could be attributed to it, as seen in other studies and case reports (12). The exact mechanism of spontaneous air leak (air leak without a predisposing factor such as mechanical ventilation) in COVID-19 is still unclear, and various theories have been proposed by different studies (13, 14). One of the most plausible theories suggests that the virus attacks mitochondria in airway epithelial cells and pulmonary artery smooth muscle cells. Mitochondria serve as not only the powerhouse of the cell but also its main consumers and sensors of oxygen (15). They control the process of programmed cell death (apoptosis) and regulate the distribution of blood flow in the lung through a mechanism called hypoxic pulmonary vasoconstriction. Another theory proposes that lung damage caused by COVID-19 results in the breakdown of the alveolar-capillary barrier, leading to air leakage into the pleural space (16). Additionally, lung damage due to dysregulation of the immune system response, inflammation, and cytokine release caused by COVID-19 can damage the alveolar walls, resulting in air space rupture during deep breathing or coughing, which can lead to air leak syndrome (14, 17, 18). Laboratory data during hospitalization showed some differences between the two groups. According to our findings, nearly all the laboratory data exhibited variances, which can be explained by the inflammatory conditions in the body due to COVID-19 infection and the inflammatory storm. We observed significantly higher total WBC counts in the CP group and a higher incidence of lymphopenia, a common condition in COVID-19, as supported by other studies (2). However, we noted significant differences in other serum biomarkers, such as LDH, Troponin, D-dimer, and CRP, which require further investigation to reinforce their validity. However, except for LDH and Troponin, these biomarkers did not demonstrate any predictive potential for complicated cases.
The CP group had a longer length of hospital stay due to the complications requiring more care. Additionally, an extended hospital stay was observed due to intubation, which could be attributed to both pulmonary complications and exacerbation of COVID-19 pneumonia.
The CP group had significantly higher mortality; however, none of the studied variables could reveal strong prediction and association. This finding underscores the importance of this relatively uncommon but significant pulmonary complication, which indeed prolongs hospitalization and may expose the patient to other issues such as hospital-acquired infections and various other events not investigated in this study but should be noted by healthcare providers. According to Chopra et al., hospital mortality among COVID-19 patients with PTX was 63%, and the odds of in-hospital death were twice as high in this group compared to COVID-19 patients without pneumothorax (19). The lower odds of death compared to the control group in their study could be attributed to their focus on patients under mechanical ventilation, among whom there is a higher likelihood of in-hospital death. Our study had several limitations. We did not differentiate between spontaneous PTX due to COVID-19 and iatrogenic PTX due to intubation. However, there were patients in the case group who were not intubated, indicating the significance of lung damage due to COVID in the development of these pulmonary complications. Additionally, this is a case-control study, and we could not study and record the parameters in real time. Furthermore, the variants of SARS-CoV-2 were not explicitly investigated.
5.1. Conclusions
Pulmonary complications of COVID-19 pneumonia worsen the prognosis in patients. The underlying process of COVID-19 pneumonia could lead to mechanical barotrauma. Additionally, our study provides additional evidence that acute phase reactants are increased in complicated cases rather than in the control group.