1. Background
Varicella-zoster virus (VZV), a member of the herpesviridae family (1), is the causative agent of two distinct clinical manifestations: Chickenpox (varicella) and shingles (herpes zoster). Primary infection with VZV causes varicella, which generally follows an uncomplicated course in healthy children. However, varicella can present serious complications in young adults and significantly immunocompromised patients (2, 3). Newborns born to infected mothers are another group at risk, as congenital malformations might develop from transplacental infection. Although the risk of congenital varicella syndrome is low, the consequences for the affected infant can be severe (4-6).
After the initial infection, the virus remains dormant and may reactivate later in life as herpes zoster, commonly known as shingles. This reactivation can cause a painful, maculopapular rash and critical neural defects, including meningitis and arterial ischemic stroke (AIS) (7, 8). Known risk factors for VZV reactivation include age, stress, and being immunocompromised (9).
The incidence of herpes zoster in the USA is estimated at 1 million cases per year (10). The World Health Organization recorded 4.2 million complicated varicella cases globally in 2014 (11). A study in Norway estimated the annual cost of varicella and herpes zoster to be nearly €9 million (12). Varicella-zoster virus is primarily transmitted through respiratory droplets from an infected individual during the contagious period of chickenpox, or through direct contact with fluid from the blisters of an individual with shingles. Once infected, individuals can transmit the virus to others who are susceptible or have not been vaccinated against it. Vaccination against varicella has significantly reduced the incidence and severity of the disease. Seroprevalence studies provide an accurate estimation of susceptible individuals, which is crucial for successful scheduling of vaccination programs (13).
Considering the estimation in different parts of the world, the seroprevalence of VZV has been reported to be 87.6%, 94.3%, and 65.8% in Korea, Turkey, and China, respectively (14-16). Few studies are available about the seroprevalence of VZV in Iran, and the results are diverse. By determining the prevalence of VZV, researchers and healthcare professionals can assess the risk of outbreaks and plan appropriate resources and healthcare services accordingly. Additionally, understanding the majority of VZV can help estimate the burden of VZV-related complications, such as hospitalizations, post-herpetic neuralgia, and other long-term sequelae, which can inform healthcare planning and resource allocation. In a systematic review and meta-analysis conducted in 2016, the overall seroprevalence of VZV infection in the Iranian population was 78.5%. The 22 studies included in the mentioned meta-analysis mainly covered central and western cities of Iran (17). No study has concerned Iran's second-largest city, Mashhad, in the northeast. Understanding the occurrence of the VZV within the Mashhad population can enhance our comprehension of the virus's epidemiology and transmission patterns. This knowledge is valuable for recognizing high-risk demographics, including age-specific prevalence rates, and identifying potential factors linked to VZV infection. To determine the prevalence of VZV in Mashhad, conducting population-based studies or surveillance programs would be valuable.
2. Objectives
We aimed to measure the prevalence of serum antibodies against VZV exclusively in 15 - 35 years-old individuals in Mashhad. The results of this study can be helpful for local health policies and improve health care services.
3. Methods
3.1. Samples Design and Study Population
This descriptive cross-sectional study was conducted in 2018 to assess the seroprevalence of VZV antibodies among individuals aged 15 to 35 in Mashhad, northeastern Iran. The population of Mashhad was approximated based on the 2006 national Iranian census. Participants were selected from three regions using a stratified cluster random sampling method, with each region divided into nine sites corresponding to the divisions of the Mashhad Healthcare Center. Eighty-two percent of individuals responded and consented to participate. All laboratory tests were performed at the Antimicrobial Resistance Research Center affiliated with Mashhad University of Medical Sciences. Informed consent was obtained from all participants before any procedures were carried out, and the study protocol was approved by the Ethics Committee of Mashhad University of Medical Sciences (code: IR.MUMS.fm.REC.1396.757).
Participants were randomly selected using cluster sampling, and demographic and biometric data were collected using a questionnaire after obtaining verbal consent. Venous blood was collected in tubes without anticoagulants, centrifuged at 3000 RPM for 5 minutes post-clotting, and stored at -20˚C. Inclusion criteria included being between 15 - 35 years old, a permanent resident of Mashhad, and consenting to participate in the study. Data collection was conducted by two certified healthcare professionals and a nurse and included demographic, anthropometric, and lifestyle data. The questionnaires covered demographic data, physical exercise, tobacco and alcohol use, a food frequency questionnaire (FFQ), and a 24-hour food record. Anthropometric measurements and cardiovascular risk-related questions were also included, along with anxiety and depression tests. Subjects undergoing treatment for rare diseases were excluded from the study.
3.2. Immunological Evaluations
Blood samples were collected from participants using a standard protocol. Each sample was centrifuged at room temperature within 30 - 45 minutes after collection to separate serum and plasma into six aliquots of 0.5 mL each, which were then sent to the Bu Ali Research Institute in Mashhad. Serological testing for anti-VZV antibodies was performed using an enzyme-linked immunoassay (ELISA). The presence of Anti-VZV IgG and IgM was assessed by reacting the antibodies in the sample with the antigen adsorbed on the polystyrene surface using commercial kits (anti-VZV IgM, anti-VZV IgG, Euroimmune, Germany). The optical density (OD) of the plates was read, and the cut-off value was calculated according to the manufacturer's instructions. There were no limitations related to the laboratory methods used.
3.3. Statistical Analysis
Chi-square tests were employed to analyze the relationship between the frequency of anti-VZV antibodies and qualitative variables such as education, employment, gender, marital status, and smoking. Fisher's exact test was used to examine the association between the frequency of antibodies and chronic diseases. Quantitative data, including age and Body Mass Index (BMI), were expressed as mean ± standard deviation (SD), and comparisons between groups were made using the t-test. Cohen’s d was utilized to calculate the effect size for the differences between groups. Logistic regression was also employed to analyze the variables. Data analysis was conducted using SPSS version 24, with a significance level set at less than 0.05 (P-value ≤ 0.05).
4. Results
4.1. General Characteristics of the Study Population
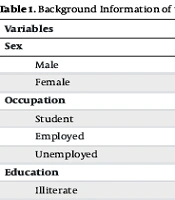
We studied 724 participants, comprising 231 (31.9%) males and 493 (68.1%) females, with a mean age of 25.52 years (SD = 5.72) and a mean BMI of 24.7 kg/m² (SD = 4.2). Participants were categorized into three occupational groups: University students, employed, and unemployed. Additionally, subjects were grouped into three categories based on marital status. The medical history of the participants indicated that 138 (19.1%) had a previous history of smoking, and 107 (14.8%) were current smokers (Table 1).
| Variables | No. (%) |
|---|---|
| Sex | |
| Male | 231 (31.9) |
| Female | 493 (68.1) |
| Occupation | |
| Student | 188 (26.0) |
| Employed | 198 (27.3) |
| Unemployed | 338 (46.7) |
| Education | |
| Illiterate | 6 (0.8) |
| Elementary | 78 (10.8) |
| Elementary to diploma | 453 (62.6) |
| Associate degree | 65 (9.0) |
| Bachelor's degree | 100 (13.8) |
| Master's degree and higher | 22 (3.0) |
| Marital status | |
| Single | 341 (47.1) |
| Married | 376 (51.9) |
| Divorced | 7 (1.0) |
| Hypertension | 17 (2.3) |
| Diabetes mellitus type | 10 (1.4) |
| Hyperlipidemia | 17 (2.3) |
| Cardiovascular Disease | 9 (1.2) |
4.2. Sero-epidemiology Assessment
The ELISA test results indicated that 85.9% of participants tested positive for VZV IgG antibodies, while 14.1% were negative. There was a significant association between the presence of VZV antibodies and age (P = 0.001), but no significant association with sex (P = 0.64). Employment status showed significant differences; 28.8% of IgG positive individuals were employed compared to 18.6% of IgG negative individuals (P = 0.02). Marital status did not significantly affect antibody presence (P = 0.11). Smoking status showed no significant difference between IgG positive and negative cases (P = 0.127). The mean BMI values were equivalent in both IgG positive and negative groups, indicating no significant differences (P = 0.97). The effect size for differences between groups was calculated using Cohen’s d and is detailed in Table 2. No significant associations were found between VZV IgG presence and a history of hypertension (P = 0.53), hyperlipidemia (P = 0.94) Among all, 2.3% of IgG-positive and 2.9% of IgG-negative participants had a history of hyperlipidemia. Lastly, the results of our study on the history of diabetes mellitus type 2 (P = 0.40), or cardiovascular disease (P = 0.46). All participants with diabetes mellitus type 2 (1.6%) and cardiovascular disease (1.4%) tested positive for IgG (Table 2).
| Total | Positive IgG Ab | Negative IgG Ab | P-Value | Effect Size |
|---|---|---|---|---|
| 622 (85.9) | 102 (14.1) | |||
| Sex | 0.64 | 0.022 | ||
| Female | 421 (67.7) | 72 (70.6) | ||
| Male | 201 (32.3) | 30 (29.4) | ||
| Age (y) | 23.8 ± 5.9 | 25.8 ± 5.6 | 0.001 b | 0 |
| Smoking | 0.127 | 0.057 | ||
| Yes | 97 (15.6) | 10 (9.8) | ||
| No | 525 (84.4) | 92 (90.2) | ||
| Employment | 0.02 b | 0.107 | ||
| Student | 151 (24.3) | 37 (36.3) | ||
| Employed | 179 (28.8) | 19 (18.6) | ||
| Unemployed | 292 (46.9) | 46 (45.1) | ||
| Marital status | 0.11 | 0.088 | ||
| Single | 284 (45.7) | 57 (55.9) | ||
| Married | 331(53.2) | 45 (44.1) | ||
| Divorced | 7 (1.1) | 0 (0.0) | ||
| BMI (kg⁄m2) | 24.7 ± 5.1 | 24.7 ± 4.1 | 0.97 | - 0.35 |
| Hypertension | 1 (1.0) | 16 (2.6) | 0.53 | |
| Diabetes mellitus type 2 | 0 (0) | 10 (1.6) | 0.40 | |
| Hyperlipidemia | 3 (2.9) | 1 4(2.3) | 0.94 | |
| Cardiovascular disease | 0 (0) | 9 (1.4) | 0.46 |
Abbreviations: BMI, Body Mass Index; SD, standard deviation.
a Values are expressed as No. (%) or mean ± SD.
b P-value < 0.05.
4.3. Relation Between Seroprevalence and Chronic Diseases
Logistic regression analysis revealed that gender (P = 0.45) and education (P = 0.67) did not significantly correlate with the level of VZV antibody. However, marital status showed a significant association (P = 0.02). No significant correlations were observed between the level of antibody and medical history, including smoking (P = 0.14), history of hypertension (P = 0.32), and history of hyperlipidemia (P = 0.30). Additionally, participants' BMI did not significantly affect antibody levels (P = 0.68).
5. Discussion
This study provides age-specific seroprevalence data of VZV IgG in the young adult population of Mashhad, aged 15 - 35. Given the scarcity of data on VZV IgG seroprevalence in Mashhad, and the outdated nature of previous studies (9, 18), this research was essential. The study found an 85.9% seropositivity rate for VZV IgG among young adults. Additionally, the study investigated the association of various factors including sex, age, smoking, employment, marital status, BMI, and chronic diseases with the presence of VZV IgG antibodies, naturally acquired through varicella infection. Analysis indicated significant associations for age and employment status. Age emerged as the most critical factor, showing a trend where IgG levels increased with age, suggesting greater exposure to VZV over time. This age-related pattern aligns with findings from other studies, such as the meta-analysis by Amjadi et al., which reported a rise in VZV seropositivity peaking at age 40 and above (17), and Vojgani et al.'s study, which also noted increased frequency of VZV IgG with age (9). These findings underscore the consistent observation of an age-dependent increase in VZV IgG seropositivity across various populations.
In this study, 85.9% of individuals aged 15 to 35 years were positive for VZV IgG. This is comparable to Swiss surveys from 1978 and 1994, which documented seroprevalence rates of 93.0% and 94.4% respectively, in adolescents and young adults (19). The gradual increase in VZV seropositivity with age mirrors patterns observed in other populations, where about 60% of children are seropositive by age four, rising to over 90% by age nine. Similarly, a study by Wutzler et al. (20) on German children and the study by Lee et al. on a Korean population also showed increasing seroprevalence with age, with near-universal seropositivity in adults over 20 years old (21). These studies highlight the global prevalence trends of VZV and underline the importance of understanding age-specific immunity to guide public health interventions.
Conversely, a recent UK study suggested a slight decline in VZV seroprevalence among adults, highlighting potential gaps in herd immunity or changes in exposure rates (22). This study, like others, faced limitations such as a restricted sample size, a common challenge in seroprevalence research that can affect the reliability of findings.
Our study demonstrated that occupation is a significant risk factor influencing VZV IgG seroprevalence, with the highest frequency of seropositive participants among the unemployed. The rate of seropositivity was similar between students and the employed group, but slightly higher among those employed. This finding can be explained by increased workplace exposures and social contacts with infected individuals, which are common in employment settings. Employed individuals may also enjoy better socioeconomic conditions that provide greater protection against infection.
The link between occupation and VZV infection remains controversial. It is recognized as one of the significant occupational hazards in the healthcare sector, particularly among susceptible individuals (23). While many infectious diseases pose risks to healthcare workers, extending these risks as general factors for the broader population requires cautious interpretation.
Furthermore, studies exploring the association between VZV prevalence and socioeconomic factors such as employment are scarce. Ghaffari Hoseini et al. conducted a multilevel analysis study that found no significant association between VZV infection and socioeconomic factors (24). Despite being a national survey, their study did not cover all age groups and needed more data from all the Iranian provinces. Furthermore, their study’s age range differed from the current study, which could be another reason for the contradictory findings about the association between VZV infection and occupation status. They measured the frequency of antibodies against VZV in the adolescent population aged 10 - 18; however, most adolescents in this age range are not employed. As a result, their study was biased and could not accurately estimate the whole population of Iran regarding seroepidemiology of VZV and risk factors.
We studied other variables, including sex, smoking, and chronic diseases. We found that the seropositive frequency in females was two times higher than in males. However, the statistical analysis showed no significance. This was likely due to the higher number of females (68.1%) in our study, resulting in a higher seropositive and seronegative frequency than males. There are some controversies regarding the association of sex with the seroprevalence of VZV. Amjadi et al. stated that the prevalence of VZV IgG is significantly higher in females and estimated an increased relative risk (RR) in women (17). In contrast, Taghavi Ardakani's study showed no significant difference between seropositive frequency regarding participants’ sex (25). However, their study was limited to Kashan city and had an age range of 1 - 15 years old, with a questionnaire about the history of exanthema filled out by the participant’s parents may have resulted in a recall bias. The study included measles, mumps, rubella, varicella, and erythema infectiosum.
About the odd ratio, the history of smoking can be considered as a risk factor for VZV infection. Our studies showed that the seropositive frequency in smokers was ten times higher than seronegative frequency. However, this finding was not statistically significant, possibly because smokers might generally be less concerned about their health and hygiene. A medical history review for chronic diseases revealed that almost all patients with hypertension were seropositive, which could be due to the small sample size of hypertensive participants. Notably, patients with hyperlipidemia exhibited a lower seropositive frequency compared to healthy participants (odds ratio < 1). Nonetheless, our statistical analysis did not find significant associations between hypertension, hyperlipidemia, and VZV IgG; thus, further research is recommended (9).
Due to the absence of the VZV vaccine in Iran's public vaccination program and the relatively high prevalence of VZV, health experts and policymakers have advocated for its inclusion in the vaccination schedule. Additionally, general screenings are considered beneficial for controlling VZV, though this approach has sparked some debate. Our study faced several limitations, such as financial constraints and insufficient serum samples, leading to a heterogeneous population and potentially skewed data. Therefore, we recommend conducting larger-scale studies to assess the association of sex, occupational status, and chronic diseases with VZV seropositivity. Further investigations in this area are necessary.
5.1. Conclusions
Our study provides insight into the epidemiology of varicella zoster among young adults in Mashhad. Regulatory organizations and policymakers can use the findings from this study to develop a local comprehensive health program and vaccination strategy for Mashhad city. We discovered that the seroepidemiology of VZV IgG is 85.9% among individuals aged 15 – 35. Given the high prevalence and contagious nature of VZV, coupled with the increasing number of immunocompromised individuals, it is crucial to maintain substantial levels of herd immunity against varicella in developed countries. Expansion of vaccination is especially recommended for family contacts of at-risk patients, healthcare workers, and women planning to conceive. Moreover, periodic studies are necessary to monitor the latest statistics and seroprevalence of VZV IgG. Conducting research with an appropriate sample size that includes all age groups is recommended to gather comprehensive data that can be generalized to the entire population.