1. Background
Since the onset of the coronavirus disease 2019 (COVID-19) outbreak caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the number of confirmed cases, associated deaths, and complications have risen rapidly, surpassing 158 million confirmed cases, with over 3 million deaths worldwide (1). Pregnant women face a higher risk of COVID-19 complications than non-pregnant women, including hospitalization, intensive care unit admission, and mortality (2-4).
Vaccination represents one of the most effective strategies to safeguard families, communities, and individuals against COVID-19. Furthermore, COVID-19 vaccines are considered a “vital tool” in managing the pandemic (5). The evidence demonstrates that these vaccines, designed to prevent severe disease, are highly efficient in reducing hospitalization and mortality linked to COVID-19 (6-8). Consequently, substantial resources have been invested in rapid vaccine development. Nonetheless, despite the availability of approved vaccines, vaccine skepticism prevails in communities worldwide, presenting an additional challenge (5). Several studies have indicated that immunity against new COVID-19 variants decreases over time in vaccinated individuals, necessitating booster doses to enhance protection. The initial dose offers substantial protection against severe illness and hospitalization due to emerging COVID-19 variants. Moreover, the booster dose further elevates protection against severe illness and hospitalization (9-11).
Vaccination during pregnancy is typically recommended to prevent complications and mortality associated with other infectious diseases, such as influenza and pertussis, for both pregnant mothers and their infants. Immunization against infectious pathogens represents one of the most effective public health measures, significantly reducing global mortality from infectious diseases (12). Concerns, whether based on factual information or misconceptions, about vaccine side effects among pregnant women can diminish their willingness to be vaccinated, potentially resulting in reduced vaccination coverage. The acceptance of vaccination during pregnancy might give rise to questions and concerns among expectant mothers (1).
The impact of vaccination on the placenta and fetus, along with the physiological changes occurring during pregnancy, renders pregnant women a unique population that might respond differently to vaccination. Safety concerns related to COVID-19 vaccines are a significant issue for both pregnant women and healthcare professionals. A survey conducted in 16 countries revealed that pregnant women displayed a higher level of hesitancy toward COVID-19 vaccination (1). In a prospective cohort study involving 1200 pregnant women in Iran, 64.8% of participants declined the COVID-19 vaccine. The most common reasons for vaccine refusal included concerns about vaccine safety and efficacy, a lack of recommendation from their obstetrician, and a preference to await additional data (13).
It is worth noting that adverse reactions and side effects can affect both pregnant and non-pregnant women. Among pregnant women, injection-site discomfort is the most commonly reported complication for both the Moderna and Pfizer-BioNTech vaccinations (14). The incidence of systemic side effects tends to increase after the second dose of the Moderna and Pfizer-BioNTech vaccines. Common systemic side effects include fatigue, headache, chills, weakness, skin rashes, and vomiting. In most cases, these complications are temporary and rarely last more than three days (15, 16).
Given the uncertainties surrounding the disease and its potential side effects, vaccination during pregnancy, particularly against COVID-19, requires careful consideration. This is essential to identify and understand possible short-term and long-term side effects, ensuring the safety and well-being of pregnant women. Therefore, the present case study aimed to investigate the possible side effects and short-term safety of COVID-19 vaccines in pregnant women in southeastern Iran.
2. Methods
2.1. Study Design
This study employed a cross-sectional design, aiming to evaluate the short-term side effects and safety of COVID-19 vaccines in pregnant women in southeastern Iran. Cross-sectional studies are valuable for describing the prevalence and characteristics of a phenomenon at a specific point in time. However, they have limitations, including potential recall bias, selection bias, and an inability to establish causality.
2.2. Study Population and Sample Size
The study population comprised pregnant women who had received various types of COVID-19 vaccines (all vaccines available in vaccination centers in Iran, such as Covaxin, AstraZeneca, Sputnik V, and Sinopharm) at the Afzalipour Medical Education Center. This center is a referral and treatment facility for COVID-19 patients in southeastern Iran, affiliated with Kerman University of Medical Sciences, Kerman, Iran, within June and August 2022. The sample size was determined using the formula for estimating a single proportion with a 95% confidence level, a 5% margin of error, and a 50% expected proportion, resulting in a minimum sample size of 384. However, to enhance the study’s power and precision, the sample size was increased by 30%, leading to a final sample size of 500. Participants were selected through convenience sampling from the list of pregnant women referred to the Afzalipour Medical Education Center for vaccination. The inclusion criteria included being pregnant, having received both the first and second doses of COVID-19 vaccines intramuscularly (0.5 mL per dose) and expressing willingness to participate in the study. The included participants were monitored for 2 weeks after receiving their first and second vaccine doses for potential side effects. Following their informed verbal consent, the mothers were interviewed by telephone to inquire about the side effects of the COVID-19 vaccines.
2.3. Data Collection and Analysis of Findings
A checklist was created in Persian by an experienced interviewer who received training from the authors of this study. This checklist encompassed questions concerning demographic characteristics, underlying medical conditions, mild and severe vaccine side effects, smoking history, and previous COVID-19 infections. An infectious disease specialist validated the checklist to ensure clarity and comprehensibility. The checklist questions were finalized following field validation.
The interviewer conducted phone interviews with the participants and collected the required information, which was then recorded in the checklists. Subsequently, another researcher reviewed the data entered into the checklists to verify its accuracy. Random follow-up calls were made to some participants to cross-check the information.
The data were analyzed using SPSS software (version 20). Descriptive statistics, including frequency, percentage, mean, and standard deviation, were employed to summarize the demographic and clinical characteristics of the participants and the vaccine side effects. The chi-square test was used to compare the frequency of side effects between different types of vaccines and between different vaccine doses. Fisher’s exact test was applied when the expected frequency in any cell of the contingency table was less than five. A p-value of less than 0.05 was considered statistically significant.
2.4. Ethical Considerations
This study received approval from the Ethics Committee of the Kerman University of Medical Sciences (IR.KMU.REC.1400.341). Additionally, informed verbal consent was obtained from the pregnant women who participated in the study. The participants were informed about the study’s objectives and significance and the confidentiality of their responses. They were assured that participation was voluntary and that there was no obligation to participate. No time limit was imposed for completing the checklist, with the average completion time being approximately 15 minutes. This study adhered to the 1975 Declaration of Helsinki (17).
3. Results
A total of 500 pregnant women participated in the present study. The participants had a mean age, height, and weight of 30.05 years, 161.67 cm, and 70.62 kg, respectively. Among the participants, 34.2% had a previous history of COVID-19 infection. Additionally, 40 participants had underlying hypothyroidism, and 13 participants were smokers.
One of the participants was a 27-year-old woman without any underlying diseases. She had received a dose of the AstraZeneca vaccine at three months of gestational age and experienced a miscarriage at 5 months of gestational age. Pathological examination (PE) suggested an interruption in the blood supply of the umbilical cord as the cause of the miscarriage. Another participant, a 38-year-old woman without any underlying diseases, received the first dose of the Sinopharm vaccine at 16 weeks and 6 days of gestational age and had a missed abortion at 20 weeks of gestational age. The investigations did not specify a particular reason for the missed abortion.
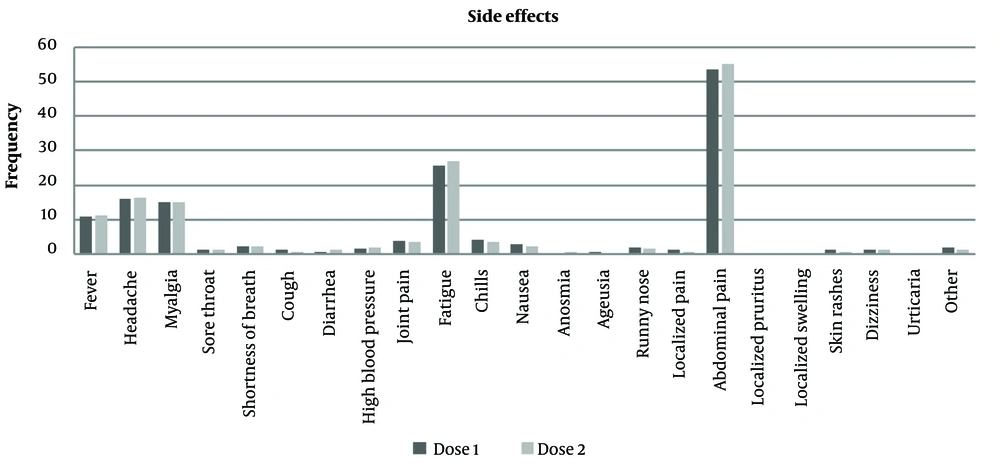
According to the results, the most common side effects of the first and second doses of COVID-19 vaccines in pregnant women were abdominal pain, fatigue, headache, and fever, with frequencies of 268 and 275, 128 and 135, 80 and 82, and 54 and 56, respectively. Conversely, the least frequent side effects of the first and second doses were localized swelling reported at 0.2% and 0%, ageusia, localized pruritus, and urticaria at 0.4%, respectively (Appendix 1, Figure 1).
Additionally, there was a significant relationship between previous COVID-19 infection in pregnant women and muscle pain associated with COVID-19 vaccines, with 39 (52%) of the pregnant women with previous COVID-19 infection experiencing muscle pain (P ≤ 0.000 and PCC ≤ 12.42) (Table 1).
| Variables | Muscle Pain; No. (%) | Degrees of Freedom | Pearson Correlation Coefficient | P-Value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Previous COVID-19 infection | 1 | 12.42 | 0.000 | ||
| Positive | 39 (52) | 132 (31.1) | |||
| Negative | 36 (48) | 293 (68.9) | |||
Abbreviation: COVID-19, coronavirus disease 2019.
The results indicated that there was a significant relationship between previous COVID-19 infection in pregnant women and post-vaccination fatigue, with 62 (45.9%) of the pregnant women with previous COVID-19 infection experiencing post-vaccination fatigue (P ≤ 0.001 and PCC ≤ 11.29) (Table 2).
| Variables | Fatigue | Degrees of Freedom | Pearson Correlation Coefficient | P-Value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Previous COVID-19 infection | 1 | 11.29 | 0.001 | ||
| Positive | 62 (45.9) | 109 (29.9) | |||
| Negative | 73 (54.1) | 256 (70.1) | |||
Abbreviation: COVID-19, coronavirus disease 2019.
Additionally, there was a significant relationship between previous COVID-19 infection in pregnant women and urticaria after vaccination, with 2 (100%) of the pregnant women with previous COVID-19 infection developing urticaria (P ≤ 0.049 and PCC ≤ 3.86) (Table 3).
| Variables | Urticaria | Degrees of Freedom | Pearson Correlation Coefficient | P-Value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Previous COVID-19 infection | 1 | 3.86 | 0.049 | ||
| Positive | 2 (100) | 169 (33.9) | |||
| Negative | 0 (0) | 329 (66.1) | |||
Abbreviation: COVID-19, coronavirus disease 2019.
4. Discussion
Coronavirus disease 2019 infection during pregnancy is associated with more severe disease and higher mortality rates. Additionally, pregnant women infected with COVID-19 are more likely to experience premature delivery, stillbirth, and other pregnancy complications. Vaccination is the most effective way to protect both pregnant mothers and their fetuses against the disease. However, concerns about potential side effects can reduce the COVID-19 vaccination rate among pregnant women. High levels of information and knowledge about the safety and effectiveness of COVID-19 vaccines, along with a reduced fear of side effects, significantly influence the decision of pregnant women, who are considered a high-risk group, to receive these vaccines (18).
The most frequently reported side effects of COVID-19 vaccines in pregnant women included abdominal pain, fatigue, headache, and fever. Conversely, the least frequent side effects were localized swelling, ageusia (loss of taste), localized pruritus (itchiness in one area), and urticaria (hives). A study conducted by Wang et al. in 2021 indicated that the most common side effects of the first dose of messenger ribonucleic acid (RNA) vaccines in pregnant women were injection-site discomfort (88.1%), fatigue (29.6%), and headache (18.1%). Side effects were more pronounced after the second dose, which aligns with the results of the present study (19).
In a study by Andrzejczak-Grzadko et al., 52.6%, 60.3%, 50.25%, 56.4%, 56.6%, and 55.6% of participants reported injection-site discomfort, shoulder pain, muscle pain, headache, and fever, respectively, which is consistent with the findings of the present study (20). Another study conducted by Elgendy et al. in Egypt reported the most common side effect of COVID-19 vaccines as injection-site redness or swelling (92%). Additionally, fatigue and lethargy (52%), fever (28%), joint pain (24%), muscle pain (20%), runny nose (8%), and dizziness, cough, allergies, rashes, convulsions, and tremors (4%) were among the other side effects reported by their study population (21).
A significant relationship was observed between previous COVID-19 infection in pregnant women and post-vaccination fatigue, with 62 (45.9%) of the pregnant women who had previously contracted COVID-19 experiencing post-vaccination fatigue. The aforementioned findings are consistent with a study that identified fatigue as one of the most prevalent side effects of COVID-19 vaccines in pregnant women (15). Moreover, a study by Pratama et al. indicated that the most common side effects of COVID-19 vaccines were injection-site discomfort, fatigue, and headache (22). Similarly, a study conducted by Syenina et al. revealed that fatigue was the most common systemic side effect of COVID-19 vaccines in pregnant women (16).
There was also a significant relationship between previous COVID-19 infection in pregnant women and urticaria (hives) after vaccination, with 2 (100%) of the pregnant women with a history of previous COVID-19 infection developing urticaria. The results of a study by Cugno et al. suggested that individuals using angiotensin-converting enzyme inhibitors are more likely to develop urticaria/angioedema after receiving the BNT162b2 mRNA COVID-19 vaccine (23). Di Ioia et al. reported that the most commonly experienced symptoms among children included urticaria (66.0%), angioedema (48.2%), pruritus (41.3%), gastrointestinal symptoms (38.1%), and breathing problems (36.8%) (24).
Similar to other studies (25), the present study did not find any evidence of an increase in the miscarriage rate. In this study, the frequency of miscarriage was reported to be 0.4% among pregnant women with underlying diseases.
This study has several limitations that should be acknowledged. Firstly, as a cross-sectional study, it cannot establish a causal relationship between COVID-19 vaccination and side effects in pregnant women; it only provides a snapshot of associations at a specific point in time. Other factors, such as maternal age, gestational age, underlying diseases, and previous COVID-19 infections, might also influence the occurrence and severity of side effects. Therefore, the results of this study should be interpreted with caution and confirmed by further longitudinal or experimental studies.
Secondly, this study might suffer from recall bias, as the participants were asked to recall the side effects they experienced after vaccination. Their memories might be influenced by their current situation, beliefs, or emotions, leading to over- or under-reporting of side effects. To reduce recall bias, objective measures, such as medical records or biological markers, could be used to verify self-reported data.
Thirdly, this study might suffer from selection bias, as the participants were selected using convenience sampling, which might not be representative of the target population. The participants who agreed to participate in the study might have different characteristics or attitudes than those who refused or were not contacted, potentially affecting the generalizability and validity of the results. Random sampling or stratified sampling could be employed to ensure a more representative sample to reduce selection bias.
The study recommends that pregnant women should be informed, encouraged, and facilitated to get vaccinated against COVID-19, and healthcare providers should monitor and report any adverse events, collaborating with other stakeholders to promote vaccination and combat misinformation.
4.1. Conclusions
The development of various vaccines and the commencement of global COVID-19 vaccination in the winter of 2020 instilled hope for saving more lives and reducing disease complications across all population groups. Nevertheless, uncertainties persist regarding the vaccines’ effects on pregnancy and fetuses due to their emergency use and the scarcity of clinical information. Additionally, immune responses to vaccines, not specifically COVID-19 vaccines, in some individuals’ bodies have created negative attitudes among the public, leading to stress. Many of these reactions can occur for reasons unrelated to the direct effects of vaccination and might be a result of the body’s immune system reacting.
Although most available COVID-19 vaccines are safe with minimal side effects, certain groups of individuals, such as pregnant and lactating women, are more susceptible to severe disease and serious complications. According to the results, the most prevalent side effects of COVID-19 vaccines in pregnant women included muscle pain, fatigue, abdominal pain, headache, and fever. Pregnant women who had previously been infected with COVID-19 were more likely to experience muscle pain, fatigue, and urticaria after receiving the vaccine. It can be concluded that all vaccines challenge the immune system, especially in pregnant women, leading to an increase in inflammatory markers and the development of side effects within a few days after vaccination. Furthermore, vaccines might cause unusual reactions in individuals with severe allergies and sensitivities. It seems that pregnant women’s medical records should be reviewed carefully before starting the vaccination program. The observation of two cases of abortion might indicate that further studies using larger samples might be needed to identify abortion as one of the complications of COVID-19 vaccination.