1. Introduction
Rabies virus, a member of the Rhabdoviridae family, is a single-stranded, negative-sense, non-segmented virus (1). It is responsible for zoonotic viral infections worldwide, with the exception of Antarctica, New Zealand, Sweden, Norway, Japan, and certain islands. Annually, 12 to 29 million people receive post-bite prophylaxis, and reported cases of casualties range from 60,000 to potentially underestimated 160,000 cases per year (2). The disease predominantly affects rural areas, with a significant burden in Asia and Africa, and it exhibits a disproportionate age distribution, primarily impacting children between 5 and 14 years of age (3).
Transmission to humans primarily occurs through contact with infected animals, and to date, human-to-human transmission has been observed only in cases where infected donors transmit the virus to organ recipients (1, 2, 4). The virus can enter the body through broken skin, mucosal surfaces, and, in rare cases, aerosols. Bites and scratches from rabid animals facilitate the virus's entry, with virus-laden saliva being the primary source (2, 5). While dogs are the principal transmission source worldwide, in regions with rigorous animal vaccination and prophylaxis, such as the United States, silver-haired and tricolored bats are the main transmission sources (6, 7).
Rabies is a fatal disease, resulting in a 100% mortality rate once symptoms develop. The central nervous system is the primary target, but extra-neural manifestations and multi-organ failure also contribute to the disease course. Acute respiratory distress syndrome (ARDS) is a rare but early extra-neural manifestation of rabies (8). In this case report study, we present a rare patient infected with the rabies virus, followed by ARDS.
2. Case Presentation
A 64-year-old male with no prior medical history presented to our emergency department complaining of sudden onset restlessness. He reported being bitten by a dog on his right leg three weeks prior to admission. He also disclosed that he had received only two doses of the post-exposure prophylaxis (PEP) vaccines and rabies immunoglobulin (RIG), which was incomplete. The patient experienced odynophagia and hydrophobia but showed no signs of aerophobia. He had jaw spasms but no seizures. The patient noticed flu-like symptoms two days before admission, which he had disregarded. Given these symptoms, a diagnosis of rabies was suspected.
Upon admission, the patient exhibited restlessness, a blood pressure of 193/101 mmHg, a heart rate of 129 bpm, a respiratory rate of 20 bpm, a peripheral temperature of 38.7ºC, and an initial oxygen saturation of 81%. His oxygen saturation increased to 94 - 95% using a facemask. A glucometer test showed a blood sugar level of 208 mg/dL. The patient was placed on continuous cardiac monitoring with pulse oximetry and was considered for intratracheal intubation.
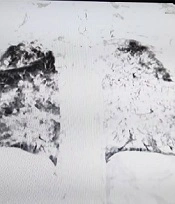
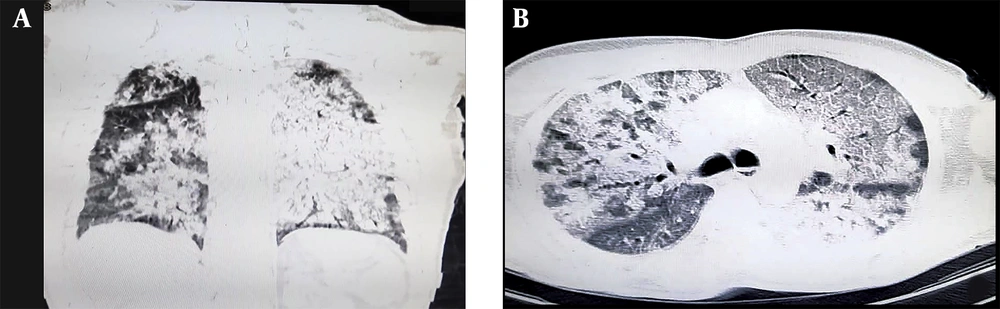
To address the restlessness contributing to the elevated blood pressure, a 5-mg dose of diazepam was administered, resulting in improved stability and a reduction in blood pressure. The patient was then evaluated for admission to the intensive care unit (ICU) and was consulted for infectious diseases. Imaging studies, including a lung computed tomography (CT) scan, were performed. These revealed near-total opacification of both lungs with consolidations, suggestive of ARDS, as well as mediastinal and parahilar lymphadenopathies with a maximum short-axis diameter (SAD) of 11 mm. An incidental finding of a hiatal hernia was also noted. Additional laboratory studies were ordered. The results of the lung CT scan and the laboratory investigations are presented in Figure 1 and Table 1, respectively.
| Parameter | Value |
|---|---|
| WBC, count/mL | 24600 |
| Lymph, % | 7.9% |
| Neut, % | 86.5% |
| Hb, mg/dL | 12 |
| Plt, count/µL | 174000 |
| Urea, mg/dL | 48 |
| Cr, mg/dL | 1.3 |
| ESR, mm/h | 14 |
| D-dimer, ng/mL | 3.04 |
| Alb, g/dL | 4.2 |
| Ca, mg/dL | 9.2 |
| Mg, mg/dL | 1.8 |
| Ph, mg/dL | 5.6 |
| VBG | |
| pH | 7.514 |
| PCO2 | 26.7 |
| HCO3 | 21.7 |
| AST, IU/L | 76 |
| ALT, IU/L | 29 |
| ALP, IU/L | 179 |
| CPK, µg/L | 1159 |
| Creatine kinase-MB, IU/L | 141 |
| Na, mEq/L | 135 |
| K, mEq/L | 3.8 |
| CRP, mg/dL | 16.5 |
| Total bilirubin, mg/dL | 1.7 |
| Direct bilirubin, mg/dL | 0.5 |
| PT, seconds | 16.6 |
| PTT, seconds | 28.3 |
| INR | 1.41 |
| Trop, ng/mL | 0.3 |
Abbreviations: WBC, white blood cell; lymph, lymphocytes; Neut, neutrophils; Hb, hemoglobin; Plt, platelet; Cr, creatinine; ESR, erythrocyte sedimentation rate; Alb, albumin; Ca, calcium; Mg, magnesium; ph, phosphorus; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; CPK, creatinine phosphokinase; Na, sodium; K, potassium; CRP, C-reactive protein; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio; VBG, venous blood gas; Trop, Troponin; IU, International Unit.
Despite the recommendation for ICU admission, no ICU beds were available. During his admission, the patient's condition improved, and he became calmer; however, dyspnea persisted. A consultation with infectious disease specialists was conducted. Due to the patient's altered mental status, bacterial meningitis and herpetic encephalitis could not be ruled out at that time. Consequently, conservative management was initiated alongside intravenous antibiotic therapy with a single dose of 2 g ceftriaxone, 1.5 g vancomycin, and 750 mg acyclovir.
Tragically, within 5 hours of admission, the patient experienced cardiac arrest. Basic and advanced life support were initiated, resulting in successful cardiopulmonary resuscitation. However, within 10 minutes, a second cardiac arrest occurred. Despite continued resuscitation attempts, the patient passed away. The patient's hourly vital signs are presented in Table 2. An autopsy was performed, and brain samples tested positive for rabies using the direct fluorescent antibody (DFA) technique. This study was conducted retrospectively from clinical data gathered during the patients' admission, and written informed consent for publication was obtained from the patients' legal guardians.
| Time | Blood Pressure, mm/Hg | Heart Rate, bpm | Respiratory Rate, rpm | Temperature, °C | Oxygen Saturation, % |
|---|---|---|---|---|---|
| Admission | 193/101 | 129 | 20 | 38.7 | 81 |
| 1 h after admission | 128/78 | 125 | 22 | 38 | 93 |
| 2 h after admission | 135/80 | 99 | 30 | 38.1 | 97 |
| 3 h after admission | 123/79 | 90 | 32 | 38 | 98 |
Abbreviations: BP, blood pressure; HR, heart rate; RR, respiratory rate; T, temperature; O2Sat, Oxygen saturation; bpm, beat per minute; rpm, respiration per minute.
3. Discussion
This study presents a compelling case of ARDS resulting from rabies following a dog bite. The array of manifestations and the involvement of various vital systems, along with a nearly total fatality rate, underscore the critical nature of rabies. This case exhibits significant involvement across multiple systems: Agitation in the nervous system, hemodynamic instability and increased blood pressure with troponin increments in the cardiovascular system, spasms and elevated serum creatine phosphokinase (CPK) levels in the musculoskeletal system, and ARDS in the respiratory system. A notable finding in this case is neutrophilia, attributable to ongoing inflammation and the patient’s stress. Additionally, the conversion from venous blood gas (VBG) to arterial blood gas (ABG) reveals apparent respiratory alkalosis with mild metabolic acidosis, which is justifiable given the patient’s condition. Although multiorgan failure (MOF) appears to be the cause of mortality, ARDS emerges as the primary contributing factor.
The rabies virus replicates after inoculation and spreads retrogradely, infecting peripheral nerve endings until it reaches the central nervous system. Studies have shown the presence of the virus in the dorsal root ganglia (DRG) within 60 - 72 hours after infection, followed by a synaptic connectivity pattern in which virtually every neuron becomes infected (1, 7). While the exact mechanism of viral entry into neurons remains unclear, studies suggest the involvement of nicotinic acetylcholine receptors. Recent studies propose that viruses can enter neurons not expressing acetylcholine receptors by using their G protein to bind with P75NTR or CD56. Microtubular transport systems within neurons may facilitate the virus's spread (1, 3, 7).
The viral incubation period varies greatly, from a few days to over 19 years. However, symptoms predominantly appear primarily within 20 - 90 days after the bite, categorizing rabies into encephalitic (furious) and paralytic (dumb) forms. The more commonly encountered rabies is the encephalitic form, accounting for 80% of cases, while the paralytic form is seen in 20% of patients, presenting a more protracted and chronic course (1, 7).
Rabies primarily affects the nervous system, and studies suggest a dysfunctional nature of the pathophysiology rather than cellular death. Involvement of brain nuclei can lead to hydrophobia, aerophobia, and autonomic system dysfunction, such as cardiac dysrhythmias, often the leading cause of death. Extra-neural manifestations include gastrointestinal disturbances, ARDS (a rare and early manifestation of rabies), and myocarditis, which may result from the hypercatecholaminemia caused by rabies (7). Table 3 presents a summary of ARDS cases secondary to rabies in the literature, highlighting its rarity.
| Author | Year | Country | Age | Sex | Primary Presentation | Location | Onset | Exposure to Presentation Time | Admission to ARDS Time | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Soler-Rangel et al. (3) | 2020 | Colombia | 25 | Female | Restlessness, dizziness, nausea, vomiting, behavioral changes | Right Arm | Progressive | 21 days | A few hours | Cat |
| Nat et al. (5) | 2012 | United States | 24 | Male | Aerophobia, hydrophobia, ataxia | - | Progressive | 8 months | 5 days | Dog |
| Hsu et al. (9) | 2006 | China | 45 | Female | Hydrophobia | Left wrist, right Leg | Progressive | 2 months | 7 days | Dog |
Viral shedding through nerve endings can result in the presence of the virus in the lungs. Following viral entry, the immune response can lead to an increase in the presence of lymphocytes, which may potentially trigger a cytokine storm. However, in our case, the occurrence of a cytokine storm was considered unlikely due to the normal levels of ESR, CRP, and d-dimer. On the other hand, some studies suggest a significant increase, up to 30-fold, in induced nitric oxide (NO) production in the brain of a rabies patient, accompanied by an elevation in reactive oxygen species (ROS) as a result of the viral P protein. Further investigation is needed to determine whether this same mechanism applies in the lungs, potentially causing the injuries and ARDS observed in the absence of heart failure (3, 7-9).
ARDS can result from various factors, with sepsis, pneumonia, trauma, transfusion, and drugs being the most common causes (10). In this case, a lung CT scan revealed bilateral near-total opacification suggestive of ARDS. However, the patient's clinical assessment and laboratory investigations did not support a diagnosis of sepsis, and there was no recent diagnosis of pneumonia, trauma, signs of alveolar hemorrhages, or a previous malignancy diagnosis. Furthermore, the patient did not report any cardiac problems before admission.
In conclusion, ARDS represents a rare yet life-threatening extra-neural complication of rabies, with cytokine storms and NO as possible etiological factors. However, further investigations are recommended to establish a more precise understanding of the pathogenesis.