1. Introduction
Nocardiosis is a rare infection caused by Gram-positive soil-borne aerobic actinomycetes of the genus Nocardia. Nocardia species are commonly found in outdoor (e.g., soil, freshwater, and marine water) and indoor environments (e.g., house dust, garden soil, and swimming pools) (1). These opportunistic pathogens mainly cause nocardiosis in immunocompromised patients, including those with a history of prolonged corticosteroid therapy, malignancy, transplantation, or human immunodeficiency virus (HIV) infection (2).
Nocardiosis may be localized or disseminated, depending on the location and extent of involvement. Most patients present with pulmonary nocardiosis. The others present with extrapulmonary nocardiosis in the central nervous system (CNS), skin, and rarely other body organs (3). Disseminated nocardiosis, which mainly originates from the involved lung, is uncommon but can be life-threatening (4).
This paper presents a case of disseminated nocardiosis with concurrent pulmonary, cerebral, and hepatic involvements in an HIV-positive patient, which may be instructive to clinicians.
2. Case Presentation
A 38-year-old woman was admitted to the Imam Khomeini Hospital (Tehran, Iran), complaining of fever, abdominal pain, productive coughs, and occasional headaches. These symptoms gradually emerged 2 months ago and progressed over time. She had also been experiencing fatigue, anorexia, diarrhea, and a significant weight loss (approximately 20 kilograms) from 6 months earlier. About 2 weeks earlier, the patient had been hospitalized in another hospital due to similar manifestations. After being diagnosed with a pyogenic liver abscess, she was treated with percutaneous drainage and intravenous antibiotics for 10 days. Furthermore, additional investigations were required because her husband was an intravenous drug user. Laboratory tests revealed that she was infected with HIV. Hence, she was referred to us for treatment.
Upon admission, the patient presented with a cachectic state, having the following vital signs: Axillary body temperature = 38.3ºC, blood pressure = 120/70 mmHg, pulse rate = 92 beats/minute, respiratory rate = 20 beats/minute, and peripheral oxygen saturation = 96% on room air. The abdominal exam revealed right upper quadrant tenderness on palpation. The other physical examinations were normal.
The results of the initial laboratory tests were: White blood cell (WBC) count = 2800 cells/mm3 with 57% neutrophils, hemoglobin = 7.2 mg/dL, platelet (PLT) count = 90,000 cells/mm3, CD4 = 46 cells/mm3, serum creatinine = 0.5 mg/dL, C-reactive protein (CRP) = 80 mg/L, erythrocyte sedimentation rate (ESR) = 54 mm/hour, aspartate aminotransferase (AST) = 17 U/L, alanine transaminase (ALT) = 10 U/L, procalcitonin = 0.2 ng/mL, blood culture×2: Negative, stool exam (WBC = 0 - 1, red blood cell (RBC) = 0 - 1), and stool culture: Positive for Isospora belli.
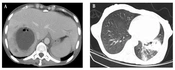
Abdominopelvic computed tomography (CT) scan with contrast demonstrated a 63 × 63 mm unilocular, non-enhancing, and hypodense lesion with ill-defined borders, with surrounding edema and air focus in the V hepatic segment, suggesting unilocular pyogenic abscess. Moreover, several non-enhancing hypodense lesions were seen in other hepatic segments (III, VI, and VIII), which probably indicated previously healed hepatic abscesses (Figure 1A). The spiral lung CT scan demonstrated evidence of consolidation in the left lower lobe accompanied by an interpulmonary abscess measuring 71 x 22 mm and centriacinar nodular opacities, suggesting lobar pneumonia with pulmonary abscess formation. Besides, numerous nodules were seen in both lungs, some of which exhibited a cavitary appearance (Figure 1B).
A, abdominopelvic CT scan with contrast, a 63 × 63 mm unilocular, non-enhancing, and hypodense lesion with ill-defined borders, with surrounding edema and air focus in the V hepatic segment; B, spiral lung CT scan, consolidation in the left lower lobe accompanied by an interpulmonary abscess measuring 71 × 22 mm and centriacinar nodular opacities.
The patient was initially diagnosed with a pyogenic liver abscess, probably caused by nocardiosis. She was empirically treated with intravenous imipenem (500 mg every 6 hours) and trimethoprim/sulfamethoxazole (3 ampoules of 400/80 mg every 8 hours). The ultrasound-guided insertion of a catheter was used to drain the liver abscess percutaneously. The obtained specimen was sent to the laboratory to explore the etiologic agent. After a few days, the reverse transcription-polymerase chain reaction (RT-PCR) test of abscess aspiration was positive for Nocardia farcinica and negative for other bacteria. In addition, bronchoscopy and bronchoalveolar lavage (BAL) were performed to identify the etiologic agent of pulmonary infection. Bronchoscopy demonstrated mucosal erythema without endobronchial lesions. Using RT-PCR, the genome of N. farcinica was detected in BAL specimens as well.
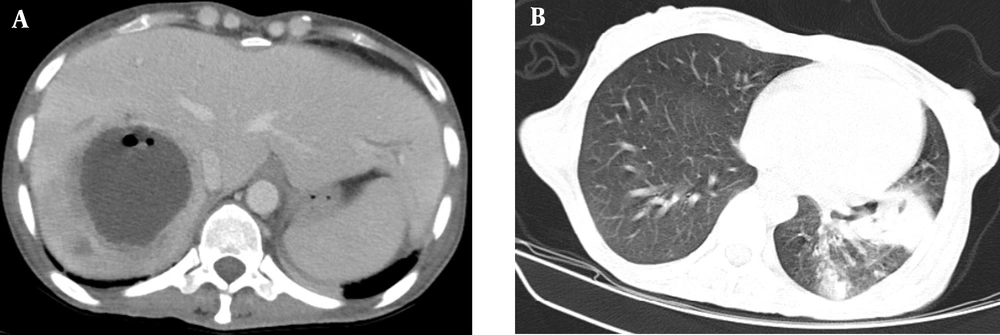
On the third day of hospitalization, the patient had seizures. Brain magnetic resonance imaging (MRI) revealed a midsagittal cortical and subcortical left light frontal convexity cystic lesion with ring enhancement and restriction diffusion, as well as surrounding perilesional edema (Figure 2A). There were also several cortical small high signal nodular lesions with similar enhancement and restriction diffusion at the right high frontal and medial right occipital and right temporal and left cerebellum, which suggested multiple bacterial abscesses formation. In addition to the administration of anticonvulsant medication, the patient underwent a lumbar puncture (CSF analysis: Glucose = 20 mg/dL, protein = 5 mg/dL, RBC = 0 - 1, WBC = 0 - 1, adenosine deaminase = 12 U/L; CSF RT-PCR test was positive for N. farcinica and negative for Mycobacterium tuberculosis).
Brain MRI, sagittal view, T2-weighted imaging. A, initial imaging, midsagittal cortical and subcortical left light frontal convexity cystic lesion with ring enhancement and restriction diffusion, as well as surrounding perilesional edema; B, follow-up imaging after 6 months; the lesions are obviously improved.
Thus, for treatment of disseminated nocardiosis with triple organ involvement, intravenous amikacin (500 mg every 12 hours) was added to the previous medication. After 3 weeks, antiretroviral therapy (ART) was initiated with oral emtricitabine/tenofovir disoproxil fumarate (200/300 mg daily) and dultegravir (50 mg daily). One week later, clinical and imaging manifestations improved, and the patient was discharged on the following oral drugs: Trimethoprim/sulfamethoxazole (3 tablets of 400/80 mg every 8 hours), amoxicillin/clavulanic acid (500/125 mg every 8 hours), and linezolid (600 mg every 12 hours).
The patient returned a week later, complaining of blurred vision in her right eye. A dilated fundoscopy of her right eye revealed spotting hemorrhages with a whitish, granular appearance on the retina, which suggested cytomegalovirus (CMV) retinitis. Therefore, 3 doses of intravitreal ganciclovir (2 mg/ injection) alongside intravenous ganciclovir (250 mg every 12 hours for 3 weeks) were injected. In addition, during the subsequent hospitalization, the patient developed progressive thrombocytopenia (up to PLT count = 13,000 cells/mm3). Thus, linezolid was discontinued, and oral moxifloxacin (400 mg daily) was substituted. After 3 weeks of treatment and subsequent absence of active retinitis in fundoscopy, she was discharged with oral ganciclovir (450 mg every 12 hours). The patient had no symptoms after a 6-month follow-up. Also, the lesions obviously improved on brain MRI (Figure 2B).
3. Discussion
We reported a case of disseminated nocardiosis caused by N. farcinica with pulmonary, cerebral, and hepatic involvements in an HIV-positive patient. According to a study by Soueges et al., N. farcinica is responsible for about 20% of nocardiosis, but it often causes the disseminated form of the disease. Furthermore, patients with disseminated nocardiosis expired more than those with localized disease (36.8% vs. 18.0%) (5). In spite of the high mortality rate in patients with disseminated form of the disease, our patient survived due to timely diagnosis and appropriate treatment.
Over 30 species of Nocardia are pathogenic to humans. The most common route of transmission is by inhalation, but it can also be transmitted through skin inoculation following direct contact with soil. Nocardia species can spread hematogenously from the lung parenchyma or from skin infections to other body organs, resulting in disseminated nocardiosis. Hematological spread of bacteria is associated with impaired macrophage function and cell-mediated immune response. Thus, immunocompromised patients are at a higher risk of developing disseminated nocardiosis (6).
Our patient had acquired immune deficiency syndrome (CD4 count < 200 cells/mm3), which predisposed her to develop a disseminated form of the disease. Based on the literature, a CD4 count below 200 cells/mm3 can indicate profound immune system impairment in HIV-positive patients, which is associated with an increased risk of developing opportunistic infections (7). Leukocytosis is anticipated to be found in individuals with an intact immune system who are afflicted with pyogenic liver abscesses, as well as an increase in serum inflammatory markers and liver enzymes (8). Our patient had leukopenia, even though her serum inflammatory markers were relatively increased, which was compatible with HIV infection.
Pyogenic liver abscess is typically caused by common Gram-negative (Klebsiella spp. and Escherichia coli) or Gram-positive (e.g., Staphylococcus spp. and Streptococcus spp.) bacteria (9). However, in HIV-infected patients, it is often caused by an opportunistic pathogen such as M. tuberculosis or Nocardia spp. (10). These pathogens can be differentiated from each other by performing culture or molecular tests on the pus aspirated from the abscess. To effectively manage a pyogenic liver abscess, the patient should receive appropriate antibiotics based on the analysis of the drained specimen (11).
Our patient displayed manifestations of pneumonia. In HIV-infected patients, pneumonia may be caused by different types of opportunistic pathogens, including bacteria (e.g., Mycobacteria spp., Nocardia spp., and Streptococcus pneumoniae), fungi (e.g., Aspergillus spp., Cryptococcus neoformans), parasites (e.g., Toxoplasma gondii and Pneumocystis jirovecii), and viruses (e.g., CMV). Therefore, detailed history taking, complete examination, and comprehensive paraclinical measures are required to discover the etiology (12). The clinical and radiological manifestations of pneumonia caused by Mycobacteria spp. and Nocardia spp. are identical. On the other hand, pulmonary tuberculosis is also more common than pulmonary nocardiosis. Consequently, some cases of nocardiosis may be initially considered to be tuberculosis. If these cases do not respond to anti-tuberculosis medications, nocardiosis should be strongly suspected. Nevertheless, it is recommended that clinicians consider both differential diagnoses from the beginning and request laboratory tests to distinguish them (13).
Most patients with CNS nocardiosis experience focal neurologic deficits, headaches, fever, altered mental status, seizures, visual disturbances, nausea and vomiting, ataxia and falls, meningism, polyuria and urinary incontinence, and personality disorders (14). However, some cases of cerebral involvement may be asymptomatic, which may have a poor prognosis (15). Clinical manifestations of CNS nocardiosis result from the mass effect of brain abscesses on the adjacent tissues (16). Like pulmonary nocardiosis, tuberculosis, aspergillosis, and toxoplasmosis are considered as the differential diagnoses of an HIV-infected patient with brain lesions (13). The diagnosis is based on microbiological assessments, including culture and molecular tests, performed on specimens obtained from lumbar puncture or abscess aspiration (17). Based on the abscess phase, it is visible as a lesion with ring enhancement on a brain CT scan or MRI, thereby enabling clinicians to investigate the etiology (16).
Treatment of disseminated nocardiosis should be initiated immediately after obtaining samples for culture and molecular tests, taking into account the extent and locations of involvement, the patient's renal function, and other medications. The following antibiotics are empirically administered as the first-line agents: Carbapenems (imipenem and meropenem), trimethoprim/sulfamethoxazole, linezolid, amikacin, and parenteral cephalosporins (ceftriaxone and cefotaxime). Disseminated nocardiosis with CNS involvement should be treated for at least 12 months, the first 3 to 6 weeks intravenously. But, in cases without CNS involvement, the treatment should be continued only for 6 months, the first 2 to 3 weeks intravenously (18).
Furthermore, the ova of I. belli were seen in the stool sample of the patient, which could explain the diarrhea. I. belli is an opportunistic parasite found in up to 20% of HIV-infected patients. It can cause severe chronic diarrhea in immunocompromised patients, leading to severe dehydration or even death (19). It is treatable with oral trimethoprim/sulfamethoxazole for 7 - 10 days (20).
During the follow-up period, our patient had some complications, like CMV retinitis. As the most prevalent ocular opportunistic infection, CMV retinitis occurs in up to 40% of HIV-infected patients. The majority of cases are asymptomatic, particularly in the early stages. Symptomatic patients complain of blurred vision, flashing lights, and floaters. Depending on the clinical variant of the disease, fundoscopic examination of patients with CMV retinitis can reveal various manifestations: Granular, white lesions on the peripheral retina with minimal hemorrhage (indolent form), yellow-white lesions with retinal hemorrhage in a sectoral, perivascular distribution (fulminant form), and opaque white lesion border (perivascular form). The diagnosis is based on symptoms and fundoscopic examination. However, PCR should be performed on aqueous or vitreous specimens to distinguish atypical cases from retinitis caused by herpes simplex virus or Toxoplasma gondii. The treatment of CMV retinitis is mainly achieved by intravenous and intravitreal injections of ganciclovir (21).
Our patient also had mild thrombocytopenia at first, which could be attributed to HIV infection (22). After the administration of linezolid, the platelet count dropped drastically. This drug was discontinued to prevent complications of thrombocytopenia. Previous studies have reported that 15 to 50% of patients develop thrombocytopenia after taking linezolid. Linezolid-associated thrombocytopenia occurs most often within the first 7 days after starting the drug. In these cases, the drug should be stopped so that the platelet count returns to the previous level within 2 weeks (23, 24).
3.1. Conclusions
Patients who are HIV-positive are particularly prone to opportunistic infections. Firstly, health care providers should consider all pathogens, even rare ones, like Nocardia spp., to establish a diagnosis if they're present. Furthermore, in cases initially diagnosed with localized nocardiosis, other body organs should also be reviewed so that the disseminated form of the disease can be diagnosed and treated immediately.