1. Background
Coronaviruses encompass an extensive variety of viruses that affect animal species. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), with manifestations varying from asymptomatic to severe acute respiratory distress syndrome (ARDS) to death (1, 2). Additionally, in severe cases of coronavirus infection, platelet damage and disturbed hemostasis are primary targets (3).
The coronavirus disease 2019 spread suddenly and caused the pandemic at the end of 2019. The disease had a high prevalence, and there was no definitive treatment. As a result, researchers have been looking for a novel and less invasive way to control the disease. However, recent reviews and meta-analyses of such treatment methods as remdesivir and nucleoside analogs, monoclonal antibodies, chloroquine (CQ) and its derivative hydroxychloroquine (HCQ), Chinese herbal medicine, convalescent plasma, and natural compounds in clinical and experimental studies have demonstrated that there is not a particular type of therapy for treating COVID‑19 (4, 5).
Low-level laser therapy (LLLT) refers to a treatment modality that employs visible light and infrared laser radiation (wavelengths of 450-1200 nm). A low-intensity laser diode (< 500 mW) emits monochromatic light or a single wavelength. Low-level laser therapy is also called cold laser therapy or photobiomodulation therapy (6).
Low-level laser therapy can be anti-inflammatory, quicken cell proliferation, and reduce pain. Low-level laser therapy effects are caused by a photochemical reaction triggered by a photo-acceptor molecule in the cell, which changes membrane permeability and metabolism. Opsins, calcium channels, cytochrome c oxidase, and water molecules are the main mediators of this process. Heat is not a factor (2). This leads to enhanced synthesis of messenger ribonucleic acid (mRNA) and cellular proliferation. Low-level laser therapy has been reported to induce the generation of reactive oxygen species (ROS) in normal cells. However, its effect on ROS levels is diminished in oxidatively stressed cells, as observed in animal disease models. Low-level laser therapy elicits an up-regulation of antioxidant defenses and a subsequent decrease in oxidative stress (7).
Published studies have revealed that LLLT controls antioxidant defenses and lowers ROS in cells under oxidative stress and animal models of disease. In pathological conditions, LLLT diminishes nuclear factor kappa B (NF-kB), which is a protein complex controlling the transcription of deoxyribonucleic acid (DNA). Numerous investigations have demonstrated abatement in reactive nitrogen species and prostaglandins in diverse animal models (8).
The anti-inflammatory properties of LLLT in the context of lung inflammation have been validated through numerous experimental animal examinations. Low-level laser therapy alleviates cytokine storms at different levels and diminishes key provocative metabolites, including interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Although IL-6 antagonists are under investigation for the treatment of COVID-19, LLLT diminishes the generation of IL-6 in addition to the production of other chemokines and metabolites (9, 10).
While stimulating tissue healing and regeneration, LLLT functions against cytokine storms and ARDS. Experimental and animal models of lung diseases and infections have shown numerous molecular and cellular effects that are local and systemic. Photobiomodulation diminishes inflammation without altering pulmonary function in acute pulmonary diseases. This treatment treats inflammatory lung complications, such as chronic obstructive pulmonary disease (COPD) (11). The use of LLLT has been proposed as a viable intervention for the management of pandemic coronavirus infections (11). The evidence from the literature supports the application of photobiomodulation to control COVID-19.
Low-level laser therapy has been utilized in the treatment of respiratory tract disorders since 1978. There are different methods for laser therapy, local and systemic, which can be referred to as whole-body irradiation or intravenous and transcutaneous methods. Intravenous laser blood irradiation therapy, also known as intravenous laser therapy or ILBI uses low levels of laser radiation to stimulate changes in the way molecules in the blood interact with each other. This therapy exposes the patient's blood to laser radiation. These changes include the interactions between lipid and water molecules, protein and water molecules, and lipid and protein molecules in the blood. The addition of intravenous LLLT of blood to conventional treatment has been shown to significantly enhance the bactericidal activity of neutrophils in cases of community-acquired pneumonia (8-10).
Low-level laser devices can be configured to target lung inflammation (12, 13). Low-level laser therapy represents fewer adverse effects and cost-effective modalities in contrast to alternative treatments and pharmaceutical agents, such as IL-6 antagonists. Low-level laser therapy has been demonstrated to be a secure, efficient, and cost-effective therapeutic modality with no observable side effects in contrast to other treatment options. Drawing from the available information, it appears that LLLT might expedite the process of convalescence from COVID-19, thereby expediting the process of getting patients off ventilator support and ultimately facilitating quicker discharge from the intensive care unit (ICU). The implementation of this measure could potentially alleviate the overwhelming burden on healthcare systems, which are currently experiencing a significant amount of tension (11).
Low-level laser therapy characteristics include the alleviation of inflammatory cytokines in cytokine storms, promotion of apoptosis of inflammatory cells, and protection of alveolar cells from damage. These findings reveal that photobiomodulation could be an effective method for the management of ARDS and can be employed with the conventional treatment of COVID-19 at various stages of the disease (11).
Therefore, considering the above-mentioned issues, the current study aimed to compare the effect of two types of LLLT, intravenous (IV) red laser and IV blue laser, on the control and recovery of COVID-19 patients.
2. Methods
This randomized clinical trial assessed the effect of intravenous lasers on COVID-19 patients in Shohada-Tajrish Hospital, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. The study protocol was approved by the Ethics on the Biomedical Research Committee of Shahid Beheshti University of Medical Sciences (registration code: IR.SBMU.RETECH.REC.1400.341) and registered in the Iranian Registry of Clinical Trials (IRCT: IRCT20111121008146N39).
A total of 60 patients who were admitted to the hospital (40 patients in the experimental groups receiving the intervention, 20 patients in the red laser group, 20 patients in the blue laser group, and 20 patients in the control group) with severe COVID-19 physical symptoms (e.g., difficulty breathing, constant pain or pressure in the chest, bluish face or lips, and sudden confusion) were included in this study. The COVID-19 patients were diagnosed with a positive RT-PCR test and radiological signs of COVID-19 on a computed tomography (CT) scan (diffuse alveolar damage). Written informed consent was obtained from all patients. The authors also explained that the information would remain confidential.
The patients with severe COVID-19 were hospitalized, and those who fulfilled the following criteria were included in this study: Saturation of peripheral oxygen (SpO2) < 93% at sea level at room temperature, a ratio of arterial oxygen partial pressure PaO2 (in mm Hg) to fractional inspired oxygen (PaO2/FiO2) < 300 mm Hg, a respiratory rate of > 30 breaths/ min, or lung infiltrates > 50% (14-16). Patients under 30 years, patients with cancer or tumor comorbidities, and pregnant patients were not included in the study.
The eligible participants were assigned to each group with a block randomization method. Block randomization was conducted to randomize the sequence. All possible patterns of blocks were written and selected randomly. In the next step, 6 patients were filled in each block (2 patients in the red laser group, 2 patients in the blue laser group, and 2 patients in the control group). Both the intervention group and the control group were treated with conventional drugs and antibiotics according to the national guidelines of Iran. In the blue and red laser groups, photobiomodulation therapy was added to the common treatment.
In the IV laser method, laser light was directly irradiated into the blood via a sterile disposable catheter. Two millimeters of optical fiber was inserted and spread into the blood circulation. This method is similar to serum injection. The evaluations were carried out once before the treatment in the treatment groups and once at the end of the treatment.
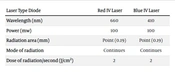
The patients were treated with a low-power gallium arsenide laser diode (660 nm) with an output dose of 2 J/cm2 for 7 minutes and 5 days in a row, similar to the same laser dose at the same time as the first group with a low-power diode laser (450 nm). Table 1 shows the laser parameters used in this experiment.
| Laser Type Diode | Red IV Laser | Blue IV Laser |
|---|---|---|
| Wavelength (nm) | 660 | 410 |
| Power (mw) | 100 | 100 |
| Radiation area (mm) | Point (0.19) | Point (0.19) |
| Mode of radiation | Continues | Continues |
| Dose of radiation/second (J/cm2) | 2 | 2 |
Patient demographic data, smoking status, alcohol abuse, comorbidity of chronic diseases, and surgery history were collected at the beginning of the study. The number of breaths/minute, heart rate/minute, blood pressure (mm/hg), body temperature (celsius), percentage of oxygen saturation at rest (mm/hg), clinical symptoms, mortality, and need for respiratory equipment, ICU hospitalization rate, laboratory tests (complete blood count [CBC], C-reactive protein [CRP], creatine phosphokinase [CPK], lactate dehydrogenase [LDH], ferritin, erythrocyte sedimentation rate [ESR], lymphocytes, IL-6), and SMART-COP which is a tool for predicting a patient with community-acquired pneumonia were assessed before and after the intervention. SMART- COP evaluates the risk of admitting the patient to the ICU by total scores. Table 2 shows COP score severity risk factors and the calculation of the severity. Total SMART-COP points could range from 0 to 11 points, with 0 - 2, 3 - 4, 5 - 6, and 7 - 11 points indicating low risk, moderate risk, high risk, and very high risk, respectively (17).
| Disease Severity Risk Factors | Points |
|---|---|
| Systolic blood pressure < 90 mmHg | 2 |
| Multiple lobes involved in the chest X-ray | 1 |
| Albumin levels less than 3.5 g/dL | 1 |
| Any age: Respiratory rate less than 25 breaths/minute | 0 |
| 50 years old or younger: Respiratory rate 25 breaths/minute or more | 1 |
| Over 50 years old: Respiratory rate 30 breaths/minute or more | 1 |
| Tachycardia of 125 beats/minute or more | 1 |
| Confusion (acute) | 1 |
| Any age: PaO2 ≥ 70 mmHg or O2 saturation ≥ 94% or PaO2/FiO2 ≥ 333 if receiving O2 | 0 |
| 50 years old or younger: PaO2 < 70 mmHg or O2 saturation ≤ 93% or PaO2/FiO2 < 333 if receiving O2 | 2 |
| Over 50 years old: PaO2 < 60 mmHg or O2 saturation ≤ 90% or PaO2/FiO2 < 250 if receiving O2 | 2 |
| pH (arterial) < 7.35 | 2 |
Data analysis was conducted using SPSS software (version 26; IBM Corp Released 2016, NY, USA). Frequency and percentages were utilized to describe the categorical variables. The mean ± standard deviation (SD) and 95% confidence interval (CI) were used to describe the quantitative variables. The normality of quantitative variables was tested with the Shapiro-Wilks test. The paired-sample t-test and the analysis of variance (ANOVA) test were employed to compare the mean differences in quantitative outcomes within and between the groups. Furthermore, the Chi-square test was used to assess categorical outcomes. The analysis was performed on completed data (without any missing data). Two-tailed tests were employed to interpret all P-values, and a P-value less than 0.05 was considered statistically significant.
3. Results
In this study, 28 males (46.7%) and 32 females (53.3%) were evaluated. The mean age of patients was 47.32 ± 11.57 years. A total of 60 COVID-19 patients were divided into three groups equally. The patients’ mean age was 45.76 ± 11.78, 44.25 ± 10.12, and 51.74 ± 9.87 in the red laser, blue laser, and control groups, respectively (P = 0.076). Male to female ratio was 10 (50%)/10 (50%) in the red group, 10 (50%)/10 (50%) in the blue laser group, and 8 (40%)/12 (60%) in the control group (P = 0.765). None of the patients in each group had a history of cardiovascular or neurologic disease. Two patients (10%) in the control group died (P = 0.126). Two patients (10%) in the control group and one patient (5%) in the blue laser group were referred to an ICU (P = 0.349).
The comparison of the fever and pulse rate before and after the study did not significantly change in each group. The evaluation of the pulmonary function of patients in each group before and after the study is shown in Table 3. The mean percentage of lung involvement had a reduction in all groups; nevertheless, 4 patients (20%) in the control group, 1 patient (5%) in the red laser group, and 2 patients (10%) in the blue laser group had severe score lung involvement after the treatment (P = 0.322). In addition, one patient in the control group had a lung involvement score increase from 40% to 60%. The mean reduction of lung involvement in the red group was more than in other groups (P = 0.001).
| Variables | Red Laser | Blue Laser | Control | P-Value |
|---|---|---|---|---|
| CT involvement | ||||
| Before | 47.00 ± 13.01 | 42.00 ± 12.39 | 42.37 ± 14.37 | 0.414 |
| After | 26.25 ± 12.02 | 29.75 ± 11.29 | 31.84 ± 17.73 | 0.665 |
| Mean difference | -20.75 ± 10.29 | -12.25 ± 4.72 | -10.53 ± 8.95 | 0.0001 |
| P-value | 0.0001 | 0.0001 | 0.002 | |
| SMART-COP score | ||||
| Before | 3.0 ± 0.65 | 2.30 ± 0.73 | 3.25 ± 0.55 | 0.0001 |
| After | 2.95 ± 0.95 | 1.75 ± 0.96 | 2.90 ± 1.16 | 0.001 |
| Mean difference | -0.05 ± 1.05 | -0.55 ± 0.99 | -0.35 ± 1.26 | 0.748 |
| P-value | 0.666 | 0.026 | 0.265 | |
| O2 | ||||
| Before | 53.69 ± 19.91 | 44.99 ± 20.93 | 58.72 ± 19.29 | 0.100 |
| After | 76.70 ± 14.88 | 65.41 ± 18.62 | 70.92 ± 15.63 | 0.104 |
| Mean difference | 23.01 ± 22.75 | 20.41 ± 22.38 | 12.20 ± 23.80 | 0.161 |
| P-value | 0.001 | 0.003 | 0.067 | |
| PO2 | ||||
| Before | 31.83 ± 17.37 | 31.09 ± 31.51 | 32.03 ± 11.69 | 0.128 |
| After | 47.44 ± 13.96 | 35.56 ± 10.44 | 41.26 ± 15.28 | 0.025 |
| Mean difference | 15.62 ± 22.17 | 4.47 ± 30.57 | 9.23 ± 20.09 | 0.161 |
| P-value | 0.002 | 0.008 | 0.067 | |
| PCO2 | ||||
| Before | 46.95 ± 7.47 | 39.70 ± 7.75 | 48.93 ± 10.81 | 0.004 |
| After | 45.51 ± 10.32 | 40.74 ± 6.19 | 50.88 ± 5.36 | 0.0001 |
| Mean difference | -1.44 ± 12.72 | 1.03 ± 8 | 1.95 ± 12.53 | 0.619 |
| P-value | 0.617 | 0.570 | 0.494 | |
| O2 Saturation | ||||
| Before | 91.76 ± 3.03 | 91.73 ± 5.27 | 90.10 ± 2.26 | 0.114 |
| After | 93.64 ± 2.78 | 93.68 ± 3.60 | 92.50 ± 3.83 | 0.544 |
| Mean difference | -1.88 ± 2.11 | -2.37 ± 3.57 | -2.95 ± 4.69 | 0.388 |
| P-value | 0.007 | 0.016 | 0.003 |
The mean COP score was reduced significantly in the blue laser group. The mean of O2 and PO2 had a significant increase in the blue and red laser groups. The PCO2 mean had a reduction only in the red laser group (-1.44 ± 12.72); nevertheless, the mean difference was not significant in three group comparisons (P > 0.05). O2 saturation decreased significantly in all groups (P < 0.05), and no significant difference was observed between the three groups. The laboratory index was compared within and between each group, as reported in Table 4.
| Variables | Red Laser | Blue Laser | Control | P-Value |
|---|---|---|---|---|
| WBC | ||||
| Before | 6.97 ± 4.22 | 6.24 ± 4.26 | 6.71 ± 3.55 | 0.480 |
| After | 9.19 ± 3.83 | 10.22 ± 3.24 | 9.25 ± 2.70 | 0.324 |
| P-value | 0.046 | 0.001 | 0.001 | |
| Lymphocyte | ||||
| Before | 17.60 ± 10.34 | 18.75 ± 8.61 | 17.80 ± 7.71 | 0.774 |
| After | 15.10 ± 7.52 | 14.80 ± 6.61 | 11.65 ± 6.09 | 0.133 |
| P-value | 0.935 | 0.141 | 0.007 | |
| PLT | ||||
| Before | 180.65 ± 62.33 | 176.95 ± 76.09 | 202.90 ± 85.67 | 0.315 |
| After | 288.70 ± 118.126 | 287.45 ± 89.70 | 275.95 ± 102.77 | 0.954 |
| P-value | 0.0001 | 0.0001 | 0.002 | |
| ESR | ||||
| Before | 22.07 ± 11.47 | 45.28 ± 28.72 | 34.0 ± 17.89 | 0.068 |
| After | 25.71 ± 14.42 | 33.50 ± 20.56 | 35.14 ± 24.74 | 0.589 |
| P-value | 0.730 | 0.045 | 0.950 | |
| CRP | ||||
| Before | 33.73 ± 19.27 | 73.17 ± 31.43 | 38.86 ± 31.41 | 0.001 |
| After | 37.01 ± 35.23 | 27.75 ± 29.27 | 39.75 ± 30.83 | 0.246 |
| P-value | 0.845 | 0.002 | 0.795 | |
| BUN | ||||
| Before | 16.95 ± 6.06 | 29.25 ± 12.72 | 19.45 ± 9.52 | 0.001 |
| After | 19.55 ± 5.59 | 38.81 ± 10.96 | 24.40 ± 12.95 | 0.0001 |
| P-value | 0.011 | 0.0001 | 0.023 | |
| Cr | ||||
| Before | 1.11 ± 0.19 | 1.01 ± 0.25 | 1.09 ± 0.36 | 0.264 |
| After | 0.96 ± 0.18 | 0.95 ± 0.19 | 1.02 ± 0.21 | 0.622 |
| P-value | 0.001 | 0.046 | 0.062 | |
| PH | ||||
| Before | 7.38 ± 0.05 | 7.40 ± 0.04 | 7.39 ± 0.05 | 0.640 |
| After | 7.36 ± 0.05 | 7.41 ± 0.03 | 7.36 ± 0.05 | 0.0001 |
| P-value | 0.093 | 0.364 | 0.019 | |
| HCO3 | ||||
| Before | 26.11 ± 2.56 | 24.44 ± 2.79 | 28.11 ± 4.32 | 0.006 |
| After | 25.28 ± 5.48 | 26.09 ± 2.77 | 29.03 ± 4.69 | 0.001 |
| P-value | 2.83 ± 6.64 | -1.65 ± 2.71 | -0.93 ± 1.33 | 0.01 |
| BE | ||||
| Before | 2.92 ± 1.72 | 1.27 ± 0.74 | 3.32 ± 2.82 | 0.003 |
| After | 3.31 ± 3.53 | 2.16 ± 1.57 | 4.20 ± 3.24 | 0.115 |
| P-value | 0.852 | 0.047 | 0.185 |
4. Discussion
The definitive treatment for COVID-19 does not exist to date. Photobiomodulation dramatically reduces the number of cells that cause inflammation and the release of chemicals that promote inflammation in the lung and reduce the amount of collagen buildup and the presence of the P2X7 receptor. Therefore, photobiomodulation is a hopeful remedy for other lung illnesses, such as COVID-19 (12). In addition, spending a long time on ventilators can harm the lungs and make the disease worse. Low-level laser therapy can be used to reduce this unwanted adverse effect. This was proven in experiments with rats, where using LLLT had a positive effect by reducing lung injury and lowering neutrophil counts in different parts of the lungs. Patients who are on ventilators need to have their inflammatory factors controlled and their healing process supported in order to be able to stop using ventilators. Therefore, LLLT is a method that is safe and noninvasive. It has been used for many years to treat pain, help wounds heal, and help with health problems, such as diseases in the lungs. Therefore, photobiomodulation, along with regular medical care, can be a good way to improve the effectiveness of treatments, lessen inflammation, help with healing, and make recovery faster (11, 17).
According to the findings of the present study, the intravascular photobiomodulation method has been used less, and this study was aimed at investigating its auxiliary effects in combination with the usual COVID-19 treatment method. Furthermore, two laser therapy groups were compared to the control group. An increase in O2 and PO2 was significant in the two laser therapy groups. In addition, PCO2 decreased significantly in the blue laser group. When CO2 constructions are low, the affinity of hemoglobin for O2 is increased. Therefore, this issue justifies the increase in O2 and PO2 in the laser group.
In three studies about the role of photobiomodulation in the treatment of COVID-19, the results of the current study regarding the positive effect of blue lasers have been investigated. The aforementioned studies show blue light positive impacts, such as the inactivation of viruses (counting coronaviruses and common flu viruses) and antibacterial effects. These impacts can be employed separately to clean, prevent the spread of coronavirus from tainted surfaces, and diminish microscopic organisms within the treatment of COVID-19 (18-20). The blue laser has multiple benefits beyond being antimicrobial and anti-inflammatory. NO is crucial for immune function, and the laser increases its production, leading to increased mitochondrial biogenesis and oxygen connection to red blood cells (21-23).
In a recent study, the COP score was reduced in all groups. In the blue laser group, the mean score before and after the treatment changed from a moderate risk (3 to 4 points) to a low risk (0 to 2 points). Moreover, the COP score in the blue laser therapy group decreased more than in the other groups (P = 0.026). However, the difference between the other two groups was not notable. It seems that laser treatment is more effective in patients who are at a certain level of severity of the disease, and it is better to stratify the severity of the disease before starting LLLT (11).
An article reported that the use of adjunctive photobiomodulation therapy in the early phases of severe ARDS observed in patients with COVID-19 could accelerate recovery and reduce the need for long-term ventilator support and ICU stay. Oxygen saturation (SpO2) rose from 93-94% to 97 - 100%, and oxygen demand diminished from 2-4 L/min to 1 L/min. Additionally, SMART-COP declined from 4 to 0. C-reactive protein normalized from 15.1 to 1.23 (10). In the present study, no mortalities were observed in the laser treatment groups. One person in the blue laser group, who had a low O2 saturation level at the beginning, was transferred to the ICU. It seems that the reason for the lack of complete recovery of oxygen saturation compared to other articles is the short duration of the study.
The white blood cells (WBC) were reduced; however, in all groups, the mean WBC increased after 5 days. Increasing the mean WBC number after a short time can be related to the involvement of other body organs, such as kidneys and the digestive system, during systemic infection.
The use of LLLT has been proven to reduce inflammation in the lungs in animals used in experiments. Photobiomodulation mitigates cytokine storm at various levels and diminishes the key inflammatory metabolites, including IL-6 and TNF-α. Interleukin 6 antagonists are being examined for the treatment of COVID-19. However, LLLT decreases the generation of IL-6 and reduces the production of other chemokines and metabolites (8, 9). Since IL-6 is one of the causes of inflammatory symptoms, such as fever, in patients and LLLT has the opposite effect on its activity, the reduction of fever in the treatment group is justified.
Research conducted on Russians has shown that IV (intravenous) treatment increases the amount of oxygen in their bodies and reduces the level of carbon dioxide. Furthermore, IV treatment stimulates oxygenation, reduces the lack of oxygen in tissues, boosts the body’s defense against infections, and regulates tissue function. Intravenous treatment decreases CRP content, increases complement activity, reduces thrombocyte aggregation ability, increases the levels of certain antibodies in the blood, and activates fibrinolysis, enhancing peripheral circulation (8). In this study, there was a significant CRP reduction in the blue laser group, and it seems that these effects might be able to justify the effects obtained from the aforementioned study (24).
There is no cure or complication-free treatment for COVID-19, as mentioned above. Therefore, any possible model that can help reduce and restore systemic or other damaged tissues can advance the treatment of adjuvant patients. More studies are required to show its effect on reducing the length of hospitalization, lung involvement, and mortality. It is suggested that the number of laser sessions and duration of laser treatment be increased in future studies.
4.1. Conclusions
In the current study, the use of an intravenous laser with red and blue wavelength with an output dose of 2 J/cm2 for 7 minutes and 5 days in a row, in addition to common treatments, showed the improvement of oxygenation (O2 and PO2 in arterial blood gas [ABG]) and the reduction of inflammatory factors (ESR and CRP) and COP scores.