1. Introduction
Progressive multifocal leukoencephalopathy (PML) is a serious and rare demyelinating disorder with high mortality caused by the John Cunningham virus (JCV), an opportunistic infection of the central nervous system (CNS). Progressive multifocal leukoencephalopathy (PML) results from the reactivation of latent JC virus infection, typically triggered by immunodeficiency. It is estimated that up to 80% of the human population is infected with the virus prior to adulthood without displaying overt clinical symptoms, remaining latent until reactivated in an immunodeficient state (1). Approximately 85% of all PML cases are associated with HIV. With the rise of the HIV pandemic, HIV infection has become the single most significant predisposing factor for PML (2). In the pre-highly active antiretroviral therapy (HAART) era, PML was diagnosed in 2 - 4% of HIV-infected patients in developed countries (3-5). Besides HIV, other forms of immunosuppression can also trigger PML. The primary medication classes suspected of initiating PML include antineoplastic agents, immunosuppressants, and corticosteroids (6-8).
Progressive multifocal leukoencephalopathy is suspected in immunosuppressed patients who exhibit focal neurological deficits. The clinical manifestations of PML are diverse, as they depend on the location and extent of CNS damage. Neurological symptoms can rapidly progress within days (9). Lesions caused by PML typically appear in the cerebral hemisphere, brainstem, and cerebellum (10-12). To our knowledge, PML in patients with AIDS is rarely reported in Iranian literature. We present a case of a patient diagnosed with HIV 4 years ago who discontinued HAART treatment and did not exhibit signs of PML or any other opportunistic infection until taking corticosteroids. Subsequently, the patient developed ataxia, and further assessment revealed PML lesions in the cerebellum.
2. Case Presentation
A 37 year-old male was admitted to the medical ward in October 2022, presenting with a 20-day history of subacute ataxia, dysarthria, and vertigo. He had no history of loss of consciousness, seizure, fever, vomiting, headache, head injury, jaundice, or ear discharge. The patient was a smoker and a social drinker, married with 2 children. His HIV test returned positive in 2019 following an admission to the hospital due to a headache, where he was diagnosed with aseptic meningitis. Highly active antiretroviral therapy (HAART) was initiated, but he discontinued the treatment just 10 days after discharge in 2019. One month prior to his current admission (June third, 2022), he was involved in a car accident, which resulted in edema in his right lower limb. He subsequently started taking dexamethasone tablets without a prescription, and 10 days later, he began experiencing ataxia, his first symptom. Upon examination, the patient was afebrile, with a pulse rate of 94/min, blood pressure of 120/80 mmHg, and O2 saturation of 97%. He was conscious and oriented and exhibited nystagmus in both eyes with slow saccades.
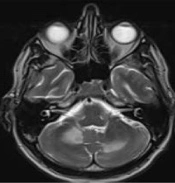
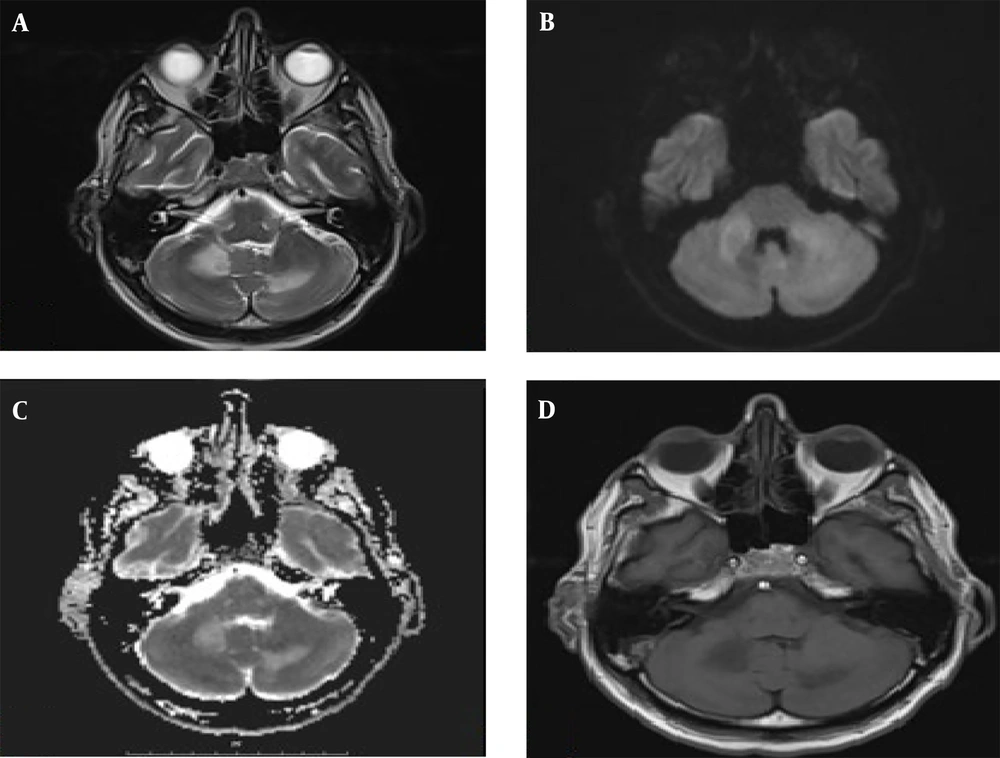
The patient's pupils were bilaterally equal and reactive to light. His speech was scanning with normal comprehension. Other cranial nerves were normal. He had normal force and sensory examination with impaired cerebellar exams on both sides. Laboratory tests conducted after admission showed WBC: 3.1, HB: 11.4, PLT: 152 000, with normal renal, hepatic, and thyroid function. The absolute CD4 count was 6.7 (5% of simultaneous TLC). Serum toxoplasma IgG was negative. Cerebrospinal fluid (CSF) analysis showed RBC: 0, WBC: 4, protein: 67, and glucose: 50, with no growth in CSF culture. The CSF was negative for cryptococcal infection and for venereal disease research laboratory (VDRL) testing. It was also negative for tubercular antigen, Epstein-Barr virus (EBV), and toxoplasma by polymerase chain reaction (PCR) in CSF. Smear and culture for routine bacteria in CSF were negative, as was the serology of CSF. MRI of the brain revealed large T2 hyperintensity involving both cerebellar hemispheres without gadolinium enhancement or edema.
Polymerase chain reaction testing for JCV in the CSF was positive. Our investigation for malignancy and paraneoplastic syndrome, which included chest, abdomen, and pelvic CT scans, as well as testicular and prostate sonography, yielded normal results. Before the confirmation of JCV PCR, the patient was started on HAART treatment along with prophylaxis for Pneumocystis jirovecii pneumonia (PCP) and toxoplasmosis. Progressive multifocal leukoencephalopathy (PML) historically has a poor prognosis; prior to the availability of antiretroviral medication, only 10% of HIV-related PML patients lived beyond one year. However, the median survival in these patients has now increased from 0.4 to 1.4 years (10, 13). Unfortunately, according to our follow-up information, the patient passed away in another hospital due to pneumonia 5 months after being discharged from our center.
3. Discussion
Neurological involvement in HIV can arise from opportunistic infections, drug toxicity, malignancy, cerebrovascular disease, or as a direct consequence of HIV infection (14). Progressive multifocal leukoencephalopathy is a type of demyelinating disease caused by the JCV, which is typically latent and becomes reactivated to infect oligodendrocytes and astrocytes. However, certain variants of the virus can also infect cerebellar granule cells and pyramidal neurons in the cortex. Progressive multifocal leukoencephalopathy affects up to 3 - 7% of patients infected with HIV-1 prior to the introduction of combination antiretroviral therapy (cART) and accounts for about 18% of fatal CNS diseases in HIV-infected patients. As a neurotropic virus, HIV can cause CNS involvement in approximately 10% of cases (15).
Progressive multifocal leukoencephalopathy usually occurs in patients with CD4+ cell counts less than 200 cells/μL (16). Corticosteroids are another cause of immunosuppression that can result in PML (7, 8, 17). While clinical features consistent with cerebral hemisphere lesions are most common, brainstem and cerebellar findings are also observed (10, 12). However, in our presented case, as illustrated in Figure 1, the lesions are located in the middle cerebellum and semioval nuclei, which are less commonly reported.
The diagnosis of progressive multifocal PML involves three stages: Clinical suspicion, radiologic evaluation (preferably using MRI), and etiologic confirmation through CSF or tissue analysis (15). Our patient, diagnosed with HIV 4 years prior, had ceased receiving HAART. He had also recently used corticosteroids. Therefore, the diagnosis of an opportunistic infection was more plausible due to the presence of two significant reasons for immunosuppression in our patient. However, our investigation also included considerations for malignancies and paraneoplastic syndromes. Notably, despite ceasing HAART treatment shortly after his HIV diagnosis, the patient did not exhibit neurological symptoms until he began using corticosteroids.
Currently, the primary therapy for PML aims to restore the host immune response to control JCV replication, as there are no specific treatments for PML. Highly active antiretroviral therapy (HAART) is the only effective therapy in this context. With HAART, the survival rate of PML patients has increased considerably, with a reported one-year survival of 39 - 56%. In HIV-infected patients not on suppressive antiretroviral therapy (ART), ART should be initiated immediately (18-20).
In HIV-positive patients with opportunistic infections, immune reconstitution inflammatory syndrome (IRIS) can occur during or after HAART as the immune system recovers. Corticosteroids have been one of the main treatments for IRIS (21). However, in the presented case and in a few other studies (7, 22, 23), corticosteroids appear to be a double-edged sword, raising the question of whether corticosteroids are a suitable option in CNS-IRIS. This issue necessitates further research to provide a definitive answer.