1. Background
Mucorales, often known as mucormycosis, include a variety of fungal strains that enter the human body through inhalation, direct contact with the skin at trauma sites, or ingestion. These strains rapidly transform from spores into invasive hyphae (1, 2). This insidious fungal pathogen, capable of attacking blood vessels, can spread through the bloodstream, leading to the involvement of multiple organs. Infections with Mucorales primarily affect individuals with compromised immune systems, resulting in increased severity and destructiveness in this vulnerable group (1, 2). Notably, patients with hematologic malignancies, organ transplant recipients, and those with diabetes are significantly more susceptible to this opportunistic infection (1, 2). The virulence of this dangerous infection varies depending on the specific strain, with Rhizopus, Mucor, and Cunninghamella, among others, identified as causative agents (3-5). Despite advances in diagnostics and treatments, mucormycosis continues to have a high impact on patient mortality (3-5).
The onset of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has resulted in an increase in reported cases of mucormycosis, drawing heightened attention from the scientific community (6, 7). The progression of coronavirus disease 2019 (COVID-19) is marked by an increased susceptibility to secondary infections, including bacterial and fungal infections. This susceptibility is due to the use of immunosuppressive medications, broad-spectrum antibiotics, and the pathophysiological traits of the viral infection itself, creating an environment conducive to secondary infectious agents (6, 7). Mucormycosis is a notable secondary infection, exhibiting a particularly high prevalence in certain areas, such as Iran (6, 7). The clinical presentation of mucormycosis depends on the host's immune status, the extent of the infection, and the affected organs. It primarily manifests as rhino-orbit-cerebral involvement, affecting the nose, eyes, and brain. However, mucormycosis can also impact other body parts, potentially affecting the lungs, kidneys, skin, or other vital organs.
In dealing with mucormycosis, early diagnosis, and prompt intervention are crucial and greatly affect patient survival outcomes. Accurate histopathological diagnosis relies on identifying broad, ribbon-like, non-septate hyphae in the affected tissues. While radiographic imaging is useful, it often falls short in providing a specific and precise delineation of mucormycosis. However, in certain cases, especially when assessing the severity and extent of the infection, the use of imaging modalities like MRI may be wisely utilized.
2. Objectives
In the following study, conducted with the necessary consent of patients or their primary caregivers, we offer a thorough analysis of clinical data and outcomes. This analysis provides insights into the complex landscape of mucormycosis post-COVID-19.
3. Methods
This clinical case-series study received approval from the ethics committees of Shahid Beheshti University of Medical Sciences. Prior to their inclusion in the study, informed consent was obtained from each patient or their legal representative.
3.1. Patient Selection
The study included individuals who were either admitted directly to the ear, nose, and throat (ENT) department of the hospital or referred for consultations from various other departments. Notably, a number of these patients had previously consulted otolaryngology specialists at other hospitals or different departments within our institution, resulting in occasional gaps in their medical records. Of the 29 patients in the study, 13 (45%) died due to the disease. Excluding 4 patients lost to follow-up for various reasons, 12 patients (41%) ultimately succumbed to the insidious fungal infection.
3.2. Clinical Assessments
The diagnosis of mucormycosis in patients was based on clinical suspicion, followed by sinus endoscopy. Key indicators were numbness, absence of bleeding, pallor, and necrosis observed during imaging. Histopathological confirmation was then pursued through biopsy. Although the majority of patients underwent diagnostic evaluations followed by combined medical and surgical interventions, some patients, unfortunately, died from the infection before any diagnostic or surgical procedures could begin.
3.3. Treatment Modalities
Patients suffering from mucormycosis, often presenting with symptoms in the oral cavity, nasal passages, eyes, and sinuses, underwent comprehensive endoscopic functional sinus surgery as part of their treatment. The purpose of this surgery was to diagnose and manage the infection and to prevent potential complications.
3.4. Surgical Procedures
The surgical techniques used in this study included careful turbinate resection to treat the affected nasal structures and, in some cases, septectomy, as necessitated by the extent of tissue involvement. The focus of the surgery was on thorough debridement, involving the removal of necrotic tissue and fungal debris, with the goal of maximum fungal eradication and preservation of healthy tissue. Of the 14 patients who underwent these surgical procedures, 11 experienced successful outcomes characterized by the resolution of the fungal infection and significant improvement in overall health. Sadly, it must be reported that three patients did not survive this aggressive treatment regimen. This outcome underscores the severe challenges presented by this relentless infection and the critical need for early diagnosis and prompt intervention.
4. Results
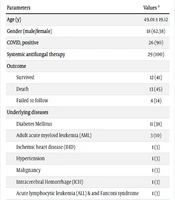
Twenty-nine cases were included in the study. The average age of the patients was 49.01 ± 19.12 years, with 18 (62%) being male. Twenty-six patients (90%) developed mucormycosis as a post-COVID-19 complication, while the remaining 3 (10%) had underlying conditions such as acute myeloid leukemia (AML) and diabetes mellitus. All COVID-19 patients had received at least 1 dose of systemic corticosteroids, and due to the severity of their COVID-19 illness, they were also treated with tocilizumab. Notably, diabetes mellitus was the most common comorbidity, present in 11 patients (38%). In contrast, 3 patients (10%) did not have any specific preexisting medical condition but developed secondary mucormycosis following COVID-19. For a detailed overview of patient demographics and characteristics, please see Table 1.
| Parameters | Values a |
|---|---|
| Age (y) | 49.01 ± 19.12 |
| Gender (male/female) | 18 (62.38) |
| COVID, positive | 26 (90) |
| Systemic antifungal therapy | 29 (100) |
| Outcome | |
| Survived | 12 (41) |
| Death | 13 (45) |
| Failed to follow | 4 (14) |
| Underlying diseases | |
| Diabetes Mellitus | 11 (38) |
| Adult acute myeloid leukemia (AML) | 3 (10) |
| Ischemic heart disease (IHD) | 1 (3) |
| Hypertension | 1 (3) |
| Malignancy | 1 (3) |
| Intracerebral Hemorrhage (ICH) | 1 (3) |
| Acute lymphocytic leukemia (ALL) & and Fanconi syndrome | 1 (3) |
| Lymphoma | 1 (3) |
| Transplant history | 1 (3) |
a Values are expressed as mean ± SD or No. (%).
A summary of the mucormycosis patient demographics and clinical characteristics is presented in Table 2.
| ID | Age | Sex | PMH | COVID | Clinical Presentation | Pathology | Surgical Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 46 | F | DM | + | Periorbital edema and decreased visual acuity | + Endo | FESS + Rt. medial Maxillectomy + Rt. PPF To ITF exposed + Septal Resection + Bilateral Turbinate resection + Rt. inferior conchae resection | Unilateral Blindness |
| 2 | 64 | M | DM | + | Palate necrosis and Lt. Facial N. palsy | + Endo | FESS + Lt. medial Maxillectomy + PPF to ITF exposed + Septectomy + Bilateral MT resection + Lt. IT resection + Lt. palate resection | Death |
| 3 | 42 | F | DM | + | Blindness | + Endo | FESS + Bilateral PPF + Bilateral MT Resection | Bilateral blindness |
| 4 | 13 | M | - | + | Periorbital edema | + Endo | FESS + Septectomy + bilateral IT resection + bilateral MT resection | Survived |
| 5 | 15 | F | Aml | + | RS + MT necrosis in Endo | + Endo | FESS + Septectomy | Survived |
| 6 | 68 | M | DM & IHD | + | RS + MT necrosis in Endo | + Endo | - | Failed to follow-up |
| 7 | 48 | F | AML | + | RS + MT necrosis in Endo | + Endo | FESS + Bilateral MT Resection | Survived |
| 8 | 60 | M | HTN & DM | + | Facial N. palsy + MT necrosis in Endo | Clinical suspection | - | Death |
| 9 | 73 | M | IHD & DM | + | Palate necrosis +columella necrosis | Clinical suspection | - | Death |
| 10 | 62 | M | - | + | Periorbital cellulitis | - | - | Failed to follow-up |
| 11 | 67 | M | IHD & Bladder cancer | + | Face necrosis | Histopathology + | - | Death |
| 12 | 60 | M | IHD | + | Periorbital edema & and palate necrosis | Endo + | - | Failed to follow-up |
| 13 | 47 | F | AML & DM | + | RS + MT necrosis | Histopathology + | Bilateral FESS + Bilateral MT Resection | Survived |
| 14 | 54 | M | AML | - | RS | Histopathology + | Bilateral FESS + Bilateral MT Resection | Survived |
| 15 | 36 | M | IHD & ICH | + | face necrosis | Histopathology +Endo + | - | Death |
| 16 | 63 | F | - | + | Periorbital swelling | Endo + | - | Survived |
| 17 | 80 | M | DM & IHD | + | Face necrosis | Histopathology +Endo + | - | Death |
| 18 | 49 | M | AML | + | RS + MT necrosis | Histopathology + Endo + | Bilateral FESS + Bilateral MT Resection + septectony | Survived |
| 19 | 66 | F | AML | + | Face necrosis | Histopathology +Endo + | - | Death |
| 20 | 34 | M | ALL | + | RS + Periorbital swelling | Histopathology +Endo + | - | Failed to follow-up |
| 21 | 37 | M | AML | + | RS + periorbital swelling | Histopathology +Endo + | Bilateral FESS + Bilateral MT Resection | Survived |
| 22 | 9 | F | ALL + Fanconi syndrome | + | MT necrosis + periorbital swelling | Histopathology +Endo + | Unilateral complete FESS (Lt.) + MT resection | Survived |
| 23 | 17 | F | Lymphoma | + | RS + MT necrosis | Histopathology +Endo + | Bilateral complete FESS + MT resection + septectomy | Survived |
| 24 | 60 | M | N/A | + | RS + MT necrosis | Histopathology +Endo + | - | Death |
| 25 | 67 | M | DM & C/S | + | Face necrosis | Histopathology + | - | Death |
| 26 | 56 | F | Renal transplantation | + | Face necrosis | Histopathology + | - | Death |
| 27 | 35 | F | DM | - | RS + MT necrosis | Histopathology + | - | Death |
| 28 | 27 | M | AML (M3) | - | RS + MT necrosis | Histopathology +Endo + | Bilateral complete FESS + MT resection | Death |
| 29 | 66 | M | DM | + | Periorbital swelling | Histopathology + | Bilateral complete FESS + MT resection | Death |
4.1. Imaging Findings
- Image 1: Depicts the involvement of the seventh cranial nerve (facial nerve) in 2 distinct patients (ID#2 and ID#8) in cases of mucormycosis.
- Image 2: Shows periorbital swelling in Patient ID#5. Computed tomography (CT) scans of the paranasal sinuses revealed extensive involvement of the frontal, ethmoidal, maxillary, and sphenoid sinuses, predominantly on the left side.
- Image 3: Displays a positive endoscopy result in a patient with maxillary turbinate necrosis.
- Image 4: Features images illustrating necrosis of the palate roof in 2 different patients.
- Image 5: Captures patients with extensive involvement of the face, eyes, nose, and sinuses, providing a clear illustration of mucormycosis, commonly known as "black fungus."
- Image 6: Offers a visual representation of periorbital swelling in Patient ID#4, who exhibited this specific symptom.
5. Discussion
Mucormycosis, primarily contracted through the inhalation of fungal spores, often begins its stealthy progression in the human body within the oral and nasal cavities. The nasal turbinates are frequently the initial site of infection. However, the aggressive nature of this fungal pathogen enables it to rapidly spread, affecting the sinuses, palate, eyes, and alarmingly, the brain by invading blood vessels, causing ischemia and subsequent necrosis (8). The global rise of the COVID-19 pandemic, which has profoundly impacted healthcare, has led to a resurgence in mucormycosis cases, commonly known as 'black fungus.' The weakened immune response due to COVID-19, compounded by the use of broad-spectrum antibiotics, steroids, and other anti-inflammatory and immunosuppressive drugs, creates an environment conducive to secondary infections. This risk is heightened in individuals with preexisting conditions like diabetes, cancer, or other immune-compromising illnesses, increasing their vulnerability to mucormycosis (9-12). As highlighted in this study, a significant number of patients with underlying medical conditions developed mucormycosis following COVID-19 infection. Clearly, the presence of an underlying condition, combined with these risk factors, plays a crucial role in the emergence of this fungal infection in COVID-19 patients.
Moreover, in line with the results of previous studies, this study found that mucormycosis predominantly affected male patients. Despite significant advancements in managing mucormycosis, the mortality rate associated with this infection remains distressingly high, consistent with statistics from before the COVID-19 pandemic (13-15).
Given the disease's rarity and poor prognosis, the optimal treatment is yet to be established. Despite various therapies, the mortality rate remains high in immunocompromised patients with mucormycosis. Prompt diagnosis and a combination of medical and surgical therapy are vital. Systemic antifungal drugs, surgical removal of the infected tissue, and management of any underlying immune deficiency are the primary treatments. The first recognized drug for this condition was Amphotericin B, and other antifungal regimens, including posaconazole and isavuconazole, are used as single or combination therapies (16).
Functional endoscopic sinus surgery (FESS) has become a prevalent method for surgically treating mucormycosis patients. In this study, a majority of patients who underwent FESS as part of their treatment showed favorable outcomes. While some research advocates for more extensive debridement and open surgical interventions, FESS has gained significant acceptance as a foundational and effective approach for initiating mucormycosis treatment. However, it is imperative for clinicians to exercise careful judgment and tailor treatment strategies as needed, shifting to more extensive surgeries when required (17-20).
Searches conducted in Pubmed, Scopus, Web of Sciences, and Ovid MEDLINE databases revealed that at least 1,336 reports on coronavirus disease–19–associated mucormycosis (CAM) were published until late 2022, using the keywords 'mucormycosis' and 'COVID-19'. A total of 958 cases from 45 different countries were analyzed (21). The common age of those affected was around 50 years, with a male predominance. India accounted for the majority of reported cases (53%), followed by the USA (10%), Pakistan (6%), and Iran (5%), alongside Mexico and France (22). Reports also came from Turkey, Austria, the UK, Italy, and Brazil. Approximately 28,252 patients in India were affected by CAM as of June 2021 (23). The most common underlying disease was diabetes (77.9%), and 78.5% of patients had a history of corticosteroid use (21). These findings align with the present study.
This study has several inherent limitations that warrant careful interpretation of the results. The relatively small sample size of 29 patients limits the generalizability of the findings, potentially not fully representing the complexity of mucormycosis post-COVID-19. The data derived from a single medical center may not reflect regional or global variations in mucormycosis epidemiology and outcomes. The lack of a control group prevents direct comparison between mucormycosis patients with and without COVID-19 history, making it difficult to attribute outcomes exclusively to COVID-19. Additionally, the short follow-up period might not capture delayed complications or recurrences, and variability in treatment practices complicates the interpretation of treatment outcomes. External factors such as socioeconomic status and evolving medical guidelines, which are not accounted for, could influence the results. The study's focus on documented cases may also introduce publication bias, potentially overlooking unreported or less severe mucormycosis cases post-COVID-19. Recognizing and addressing these limitations in future studies is essential for a deeper understanding of this complex clinical scenario.
5.1. Conclusions
This study highlights the complexity of mucormycosis following COVID-19, considering factors like immunosuppression, comorbidities, and therapeutic interventions. Prompt diagnosis and early intervention are critical due to the disease's potential for rapid and life-threatening progression. The observed predominance of male patients is consistent with previous findings. The notable mortality rate underscores the need for improved treatment approaches. Functional endoscopic sinus surgery (FESS) proves beneficial, though its timing and extent require tailoring to each patient. The study's limitations, including its modest sample size and potential biases, call for further research. Effective management of mucormycosis necessitates a multidisciplinary approach, increased awareness, and preventive strategies.