1. Background
There are various coronaviruses worldwide, and while there is no definitive treatment, prevention is crucial (1), with COVID-19 vaccines being a vital intervention (2). These vaccines are highly effective against symptomatic and severe diseases caused by the main variant of SARS-CoV-2, including the alpha variant (B.1.1.7) (3). However, due to limited and conflicting clinical evidence, the efficacy of the vaccines is still a subject of debate (4). The World Health Organization (WHO) emphasizes the importance of following health protocols despite ongoing research on vaccine protection against disease, infection, and transmission (5). Studies by the Centers for Disease Control and Prevention (CDC) in the United States show that, among 101 million vaccinated individuals, approximately 10,262 experienced COVID-19 reinfection, with 995 hospitalizations and 160 deaths, highlighting the effectiveness of vaccination despite not providing 100% immunity (6).
Vaccinating children is considered crucial in combating the epidemic, as achieving herd immunity is unlikely without vaccinating them, even if a large proportion of adults are vaccinated (7). Studies on vaccine side effects in children under 18 have reported issues such as myocarditis, myopericarditis, injection site swelling, and headache (8). In Iran, the vaccination of high school seniors with the Sinopharm vaccine began in September 2021 (8). The CDC recommends COVID-19 vaccination for individuals aged 12 and above as a crucial measure to protect against the disease and help curb the epidemic (9).
2. Objectives
Given the lack of sufficient evidence on available vaccines in Iran and the ongoing phase three research, data is needed to assess vaccine effectiveness among different populations and inform public health policies and recommendations. Therefore, considering the reopening of schools and the importance of the student’s health, this study aimed to determine the potential side effects and symptomatic reinfection rates in the population aged 12 - 18.
3. Methods
3.1. Study Design and Population
This longitudinal study was carried out in Dezful, Iran, from 2021 to 2022, which is located 721 km southwest of Tehran. The study included individuals aged 12 - 18 years who received the first dose of COVID-19 vaccines (Sinopharm and PastoCovac) at comprehensive health service centers in 2021, as recommended by the Ministry of Health and Medical Education (MOHME). The study followed their vaccination feedback for 6 months to assess side effects and post-vaccination reinfections. The targeted population size for the studied age groups, as reported by Dezful Health Center in 2021, was 39,963. Sample size calculation determined a sample of n = 380.
In order to take into account the probable dropout of the subjects of the study during the six months of follow-up, a larger sample size (i.e., 1000 people) was considered. The samples were selected using random systematic sampling. After identifying all the people of the target group, which were about 40,000 people, and considering 1000 people, the size of the study sample and the interval between the samples were determined as follows:
The interval between samples = Number of samples ÷ number of people in the target group
40000 ÷ 1000 = 40
Then, a random draw was made between the numbers 1 to 40. The first determined number was considered as the first person, and the next 999 people were selected at an interval of 40 people. Given the national code extracted from the ‘SIB system,’ a code was allocated to each of the designated numbers. Based on the coordination made, 1000 specified codes were provided to all urban and rural comprehensive health service centers responsible for the COVID-19 vaccine (prior to the start of nationwide vaccination for those under 18 years of age in Iran). When referring to health centers, the researchers were provided with the mentioned codes to fill in the forms. Following interviewing the samples referring to the health centers and explaining to them the goals of the project, in case they met the required criteria for entering the study and if they were willing to cooperate, the consent form was presented to be filled in and signed by them, and their code was included in the study. In case the specified samples were not willing to participate in the study or if they did not visit the researcher, the necessary replacement was done by maintaining the sample interval.
The study was designed as a longitudinal study with the specific aim of following the conditions of individuals who had received the vaccine.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria of this research were:
• Individuals between 12 and 18 years old (born in 2003 - 2009).
• Those referred to comprehensive health service centers and received shots of the COVID-19 vaccine.
• Participating in the study with consent while being fully informed.
• Cooperation and self-declaration of requested questions for 6 months.
The exclusion criteria of this research were:
• Not agreeing to participate in the study for 6 months.
• Failure to answer researchers’ questions.
• Not responding to phone calls.
• Individuals’ death for any reason.
• History of anaphylactic reaction after receiving any vaccine.
People who had an anaphylactic reaction following the injection of the vaccine were excluded from the study, but their information was recorded.
3.3. Data Collection
Interviewers visited comprehensive health service centers and collected data by filling out the demographic characteristics checklist (within the framework of an electronic questionnaire). Then, the samples were actively and passively traced for 6 months (according to the conducted studies) (10). Given the side effects caused by the vaccine and symptomatic reinfections (i.e., clinical or radiological evidence compatible with COVID-19 with the approval of a medical specialist or positive PCR test) following injection of the first and second dose of the vaccine were followed up, and their information was recorded in separate checklists. Participants with symptomatic reinfections were those who, after receiving the vaccine, experienced symptoms consistent with COVID-19, tested positive on a PCR test, or exhibited signs of the disease along with confirmed pathological findings by the relevant specialist and study focal point. It is noteworthy that their PCR test could also yield a negative result. In order to determine the side effects caused by the vaccine, active follow-up of samples was performed via phone calls and self-declaration, and all the information of this stage was entered in the checklists of side effects of the first and second doses. Finally, all recorded side effects and problems of the studied subjects were recorded, followed up, and analyzed, and the procedure continued until the symptoms were resolved. Also, the samples who were infected following the injection of the first and second doses of the vaccine were questioned every two weeks, and the results obtained were recorded in the relevant checklists. In addition, at the end of each day, after recording the data of the samples in the electronic questionnaire, the output of the data was determined. It is noteworthy that the research team gave the samples the necessary instructions to eliminate the side effects or diagnose and treat the reinfections after the injection of the vaccine. Six months later, the data obtained was analyzed. It should also be noted that symptomatic reinfections, rather than asymptomatic ones, were the focus of the study. Therefore, the study was conducted to identify symptomatic reinfections.
3.4. Data Analysis
Descriptive analyses were conducted to calculate the distribution of sociodemographic characteristics, clinical characteristics, and epidemiological factors of COVID‐19 patients. The data related to continuous variables were reported as means and standard deviations, and categorical data were reported as frequency and percentages. The McNemar test has been used to compare post-vaccination side effects in the interval between the first and second doses.
Logistic regression models were used to determine the relationship between demographic characteristics, the type of vaccine received, the risk of experiencing vaccine-related side effects, and COVID-19 reinfections after vaccination. All statistical analyses were conducted using SPSS 24. The statistical significance was defined as a p-value less than 0.05.
4. Results
It is noteworthy that 1000 individuals were enrolled in the study and remained in the study until the end of the 6 months, and no participants dropped out.
4.1. Side Effects of COVID-19 Vaccination
Analysis of the obtained data indicated that 53.1% of vaccinated people were male and, 46.9% of them were female (n = 1000), 31.4% were between 12 and 15 years old, 23.3% were 16 years old, 25.9% were 17 years old, and 19.4% were 18 years old. Regarding the level of education, 40.5% had elementary and junior high school education, and 59.5% had senior high school education. As for vaccines used, 7.6% were vaccinated with the PastoCovac vaccine and 92.4% with the Sinopharm vaccine; 9.6% of the people had a history of chronic diseases, and 17.6% had a history of allergies. Based on the reports, 31.3% of the cases declared they had COVID-19 reinfections prior to vaccination, 11.6% cases reported COVID-19 reinfections after receiving the first dose of the vaccine, and 14.3% cases reported being infected following the injection of the second dose of the vaccine. The frequency of side effects after receiving the first and second doses of the vaccine is tabulated in Table 1. As shown in Table 1, pain at the injection site was the most common complication after receiving the first dose, with a frequency of 25.1%. Skin rash was the least frequent at 0.4%, and regarding complications after the second dose, pain at the injection site was the most frequent at 17.2%, and skin rashes at 0.1% have had the least frequency. In addition, 39.9% of people mentioned having at least one side effect after injection of the first dose and 27.2% after the injection of the second dose of the vaccine.
| Side Effects of The Vaccine | No (%) | P-Value | |
|---|---|---|---|
| First Dose | Second Dose | ||
| Headache | 121 (12.1) | 80 (8) | <0.001 |
| Body ache (myalgia) | 114 (11.4) | 94 (9.4) | 0.02 |
| Gastrointestinal discomforts | 17 (1.7) | 11 (1.1) | 0.14 |
| Fatigue | 226 (22.6) | 148 (14.8) | < 0.001 |
| Stiffness of injection site | 133 (13.3) | 94 (9.4) | < 0.001 |
| Pain in the injection site | 251 (25.1) | 172 (17.2) | < 0.001 |
| Losing sense of smell | 23 (2.3) | 13 (1.3) | 0.05 |
| Losing sense of taste | 22 (2.2) | 9 (0.9) | < 0.001 |
| Impaired consciousness | 11 (1.1) | 7 (0.7) | 0.22 |
| Nasal congestion | 36 (3.6) | 20 (2) | < 0.001 |
| Runny nose | 45 (4.5) | 24 (2.4) | < 0.001 |
| Shortness of breath (dyspnea) | 20 (2) | 13 (1.3) | 0.09 |
| Allergic reactions | 8 (0.8) | 4 (0.4) | 0.22 |
| Anxiety | 28 (2.8) | 20 (2) | 0.07 |
| Phobia | 20 (2) | 16 (1.6) | 0.42 |
| Appetite reduction | 43 (4.3) | 24 (2.4) | 0.001 |
| Vertigo | 74 (7.4) | 52 (5.2) | 0.002 |
| Sleepiness | 129 (12.9) | 82 (8.2) | < 0.001 |
| Cough | 22 (2.2) | 14 (1.4) | 0.13 |
| Sneeze | 35 (3.5) | 17 (1.7) | 0.001 |
| Sore throat | 25 (2.5) | 13 (1.3) | 0.01 |
| Nausea and vomiting | 21 (2.1) | 16 (1.6) | 0.36 |
| Fever | 79 (7.9) | 40 (4) | < 0.001 |
| Chills | 34 (3.4) | 20 (2) | 0.01 |
| Chest pain | 12 (1.2) | 7 (0.7) | 0.22 |
| Backache | 20 (2) | 12 (1.2) | 0.06 |
| Skin lesions/rash | 4 (0.4) | 1 (0.1) | 0.25 |
| Having at least one complication after vaccination | 399 (39.9) | 272 (27.2) | < 0.001 |
4.2. Chances of Side Effects After Vaccination
The results of the regression analysis indicated that the chance of having any complication emanating from the injection of the vaccine after the first and second doses in women was 2.34 and 1.91 times higher than in men, respectively. Also, people who had a history of allergy and underlying diseases had a 1.63- and 1.87-times higher chance of any complication caused by the vaccine after injection of the first dose, respectively. In addition, the chance of having any complication caused by the vaccine after the first dose was 1.99 times higher in those vaccinated with the PastoCovac vaccine than among people who had been vaccinated with the Sinopharm vaccine. It is worth noting that the chance of experiencing vaccine side effects after the first dose increases by 1.14 times with each additional year of age. Also, the chance of having any complication caused by the vaccine after injection of the second dose was 0.55 among people with elementary and junior high school education levels (Table 2).
| Characteristics | First Dose | Second Dose | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| Gender | ||||||
| Male (Ref) | 1 | 1 | ||||
| Female | 2.34 | 1.79 - 3.05 | <0.001 | 1.91 | 1.43 - 2.56 | <0.001 |
| Education level | ||||||
| Senior high school (Ref) | 1 | 1 | ||||
| Elementary and junior high school | 0.84 | 0.56 - 1.24 | 0.37 | 0.55 | 0.35 - 0.85 | 0.007 |
| History of allergy | ||||||
| No (Ref) | 1 | 1 | ||||
| Yes | 1.63 | 1.15 - 2.34 | 0.007 | 1.65 | 0.92 - 1.96 | 0.12 |
| Presence of chronic disease | ||||||
| No (Ref) | 1 | 1 | ||||
| Yes | 1.87 | 1.18 - 2.97 | 0.008 | 1.51 | 0.94 - 2.41 | 0.08 |
| Type of COVID-19 vaccine | ||||||
| Sinopharm vaccine (Ref) | 1 | 1 | ||||
| PastoCovac vaccine | 1.99 | 1.22 - 3.27 | 0.006 | 0.91 | 0.51 - 1.62 | 0.75 |
| Age | 1.14 | 1.01 - 1.29 | 0.04 | 1.07 | 0.93 - 1.22 | 0.35 |
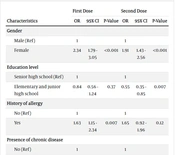
Also, the results of the regression analysis indicated that the chance of being infected by COVID-19 after receiving the first dose of the vaccine was 1.65 times higher among women than men. With an average increase of one year of age, the chance of contracting COVID-19 after receiving the first dose was 1.29 times higher. Moreover, there was no significant relationship between background variables and types of injected vaccines with the reinfections of COVID-19 after injection of the second dose (Table 3).
| Characteristics | First Dose | Second Dose | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| Gender | ||||||
| Male (Ref) | 1 | 1 | ||||
| Female | 1.65 | 1.12 - 2.46 | 0.01 | 1.18 | 0.83 - 1.70 | 0.36 |
| Education level | ||||||
| Senior high school (Ref) | 1 | 1 | ||||
| Elementary and junior high school | 1.32 | 0.75 - 2.32 | 0.33 | 0.82 | 0.47 - 1.43 | 0.48 |
| History of allergy | ||||||
| No (Ref) | 1 | 1 | ||||
| Yes | 1.51 | 0.92 - 2.46 | 0.10 | 1.21 | 0.76 - 1.91 | 0.43 |
| Presence of chronic disease | ||||||
| No (Ref) | 1 | 1 | ||||
| Yes | 0.93 | 0.48 - 1.77 | 0.83 | 1.61 | 0.93 - 2.78 | 0.09 |
| Type of COVID-19 vaccine | ||||||
| Sinopharm vaccine (Ref) | 1 | 1 | ||||
| PastoCovac vaccine | 1.32 | 0.64 - 1.70 | 0.44 | 0.43 | 0.17 - 1.08 | 0.07 |
| Age | 1.29 | 1.07 - 1.54 | 0.006 | 1.03 | 0.87 - 1.23 | 0.67 |
5. Discussion
Many studies on the effectiveness and side effects of COVID-19 vaccines in adults have already been conducted and published. However, few studies have focused on people under 18 years old due to ethical considerations and the delays in issuing the necessary licenses. Numerous studies have reported several side effects resulting from COVID-19 vaccination. The most common side effects include local side effects such as pain at the injection site, mild fever, headache, weakness, and myalgia. The results of our study are in agreement with those of previous reports (11-16). Most of these side effects emerged within the first 72 hours following the vaccination.
Fifty percent and 39% of people reported side effects after receiving the first dose of Soberana (PastoCovac) and Sinopharm vaccines, respectively, and 22% and 27% after receiving the second doses of the vaccines. According to the results of similar studies, side effects seem more common after the first dose of the vaccine due to immune response (1, 5).
It appears that the side effects experienced by adults are similar to those detected in people who are under 18 years old (17). Also, this paradigm does not depend on the type of vaccine (mRNA vaccines or non-mRNA vaccines) because the common side effects of all COVID-19 vaccines are almost the same (18). The majority of participants in the study received the Sinopharm vaccine, accounting for over 92% of the study group. This was due to the fact that at the time of the study, only two vaccines, Sinopharm and PastoCovac, had been granted a license by the Iran Food and Drug Administration (IFDA). This decision was based on the best available evidence, as well as the greater availability of the Sinopharm vaccine (19).
The results of the study showed that the emergence of severe side effects following injection of these two vaccines among people under 18 was rare. In a study conducted by Boshra et al., two vaccines (i.e., Sinopharm and AstraZeneca) were compared. The incidence of side effects was lower after injection of the Sinopharm vaccine, and it was declared as a safe vaccine, although all participants were above 18 (20). Also, in a study conducted in Pakistan on the Sinopharm vaccine, no severe or life-threatening side effect was reported (21).
We observed that the chances of side effects of these two vaccines among people aged 12-18 years are higher in women than in men (OR = 2.3, 95% CI: 1.79 - 3.05), and it was higher among people who had a history of allergies or underlying diseases (OR = 1.63, 1.87, respectively). In a study conducted by Babaee et al. in Iran on people above 18, it was reported that more than 54% of people who had side effects due to injection of the Sinopharm vaccine had at least one underlying disease. The most common underlying disease was diabetes. Interestingly, similar to our study, the incidence of side effects was higher in women (22). In another study in Bangladesh, it was reported that people with underlying diseases had a higher risk of developing side effects caused by the COVID-19 vaccines (23). It seems that following the injection of these vaccines, women and people with underlying diseases should receive more attention and follow-up.
Following the injection of the first dose of vaccines, 11.5% and 13.2% of vaccinated people (Sinopharm and PastoCovac, respectively), and up to six months after receiving the second dose, 14.9% and 6.6% of persons were re-infected. Also, 26.3% of them had a history of at least one episode of SARS-COV-2 infection prior to receiving both the Sinopharm and PastoCovac vaccines. The results of previous studies were different based on the study population and the type of vaccines used (24). In a study in Iran conducted by Tavakoli et al. on people under 18, only 1.8% of persons developed a COVID-19 infection after injection of two doses of the vaccine (i.e., PastoCovac or Sinopharm) (17). In our view, this difference can be attributed to various reasons. The first one is the time of follow-up. In the mentioned study, the follow-up period was three weeks, and in our study, it was 6 months. Secondly, the epidemic waves of the disease and exposure cases definitely affect the frequency of new or re-infected cases. At the time of the study, we were experiencing a new wave of COVID-19 epidemic peak. Thirdly, the genetic changes of the virus and the creation of new variants are effective in the number of infections (25, 26). Finally, over time and with the wane of vaccine immunity, the risk of the disease increases (20).
It seems that the previous immune status (naturally infected populations, infection-naïve, and vaccinated individuals) is also effective in the risk of re-infection (27, 28). We found that the reinfection rate within 6 months after vaccination was lower in naturally infected populations than in infection-naïve peoples (17.5% vs 20.8%). The risk of infection after vaccination is lower in naturally-infected populations, but this hypothesis should not be the basis for vaccination. Recent research has shown that severe and frequent infections with SARS-COV-2 may increase the risk of long-term complications (29, 30). Therefore, we should prevent people from getting infected with the SARS-COV-2 virus frequently, and one of the most effective ways is mass vaccination.
The mortality is higher among the elderly and adults with underlying diseases, but due to the delay in vaccination of those under 18, this probability has shifted towards children (31). Studies conducted on vaccinated children are limited. Taking into account various factors, parents’ willingness to vaccinate their children is about 61.4% (21.6% to 91.4%), but it seems that the actual rate of child vaccination is smaller (32). Most studies emphasize the necessity of vaccinating children, especially those with underlying diseases. One of the main goals of vaccination in this group is to prevent serious complications such as multisystem inflammatory syndrome (MIS-C) (33).
In a study conducted by Yadegarynia et al. in Tehran, the impact of three COVID-19 vaccines (AstraZeneca, Sputnik V, and Sinopharm) was evaluated on 377 healthcare workers. The results indicated that the risk of side effects after injection of the first dose of the vaccine was higher than that of the second dose. Also, the most common side effect caused by the vaccines was local reactions. The least common side effects were seen with the Sinopharm vaccine. Although the participants in this study were not the same in our research, the results were similar. We assume that due to sensitization of the immune system after the injection of the first dose of vaccines, the chance of side effects will decrease with subsequent doses. This scenario seems to be the same for all COVID-19 vaccines. Based on the results of different studies, we figured out that the Sinopharm vaccine has the lowest probability of serious side effects compared to the other COVID-19 vaccines (34).
The keywords COVID-19, vaccine, and side effect, as well as the Google Scholar, Scopus, and PubMed databases, were searched, and the following studies were found. The most common side effects of COVID-19 vaccines in individuals under 18 are shown in Table 4.
| Author | Year | Country | The most Common Side Effects | COVID-19 Vaccine |
|---|---|---|---|---|
| Frenck et al. (35) | 2021 | USA | Injection-site pain | Pfizer BioNTech |
| Creech et al. (36) | 2021 | USA, Canada | Injection-site pain, headache, and fatigue | mRNA-1273 vaccine (Moderna) |
| Thomas et al. (10) | 2020 | Argentina, Brazil, Germany, South Africa, Turkey, USA | Local reaction | Pfizer BioNTech |
| Munoz et al. (37) | 2022 | USA, Brazil, Poland, Spain, Finland. | Local reaction | Pfizer BioNTech |
| Tian et al. (38) | 2022 | China (systematic review) | Injection-site pain, fever, headache, cough, fatigue, and muscle pain | Inactivated virus vaccines, viral vector vaccines, RNA vaccines, DNA vaccines, recombinant vaccines, subunit vaccines |
| Walter et al. (39) | 2021 | USA, Spain, Finland, Poland | Local reactions | Pfizer BioNTech |
| Ali et al. (40) | 2021 | USA | Injection-site pain | mRNA-1273 vaccine (Moderna) |
| Han et al. (41) | 2021 | China | Injection-site pain | CoronaVac inactivated vaccine |
| Xia et al. (19) | 2020 | China | Local reaction | BBIBP‐CorV Inactivated vaccine |
| Zhu et al. (42) | 2020 | China | Injection-site pain, fever, headache, and fatigue | Ad5‐nCoVAdenovirus vaccine |
| Khobragade et al. (43) | 2021 | India | Injection-site pain, fever, headache | ZyCov‐D DNA vaccine |
Currently, due to a lack of evidence, the consequences of COVID-19 disease are not fully known, and further studies are needed. On the other hand, the pandemic of COVID-19 has changed to an endemic status, and it does not seem that this virus will eradicated in the near future, and it continues to be a public health threat. Therefore, it is necessary to improve vaccination coverage for children and develop potent vaccines for various virus variants (44).
5.1. Limitations of the Study
There existed a few limitations in this study:
- It was difficult to follow this number of people for six months, but we solved this problem by increasing the number of research team members and training them.
- Several factors have been influential in the results of studies conducted on vaccines. Because it was not possible to evaluate all factors in this research, more studies are needed to investigate various aspects of vaccination among people aged 12 - 18 years.
- We intended to evaluate more vaccines, but at the time of conducting this study in Iran, only two vaccines, PastoCovac and Sinopharm, were licensed for people aged 12 - 18 years. Therefore, it is our recommendation to use a control group in future studies, as one was not included for the reasons previously mentioned.
- Due to the limited resources (financial and human), despite the desire of the research team, it was not possible to increase the number of participants in the study.
- Another limitation of the study was the limited number of individuals vaccinated with the PastoCovac vaccine, which was unavoidable. Furthermore, due to the unavailability of specific samples, the vaccine was chosen, and as a result, we had to include the results and compare them with the Sinopharm vaccine. Ideally, it would have been preferable to have roughly the same number of vaccinated individuals in each group under similar conditions. However, we acknowledge that this was not feasible.
Due to the passive follow-up of the study, from the third day onwards and every two-week follow-up and the individual’s self-report, even though the subjects were told to contact the researcher in case of the emergence of any symptoms, they might have missed such an opportunity.
5.2. Conclusions
The results of the study demonstrated that Sinopharm and PastoCovac vaccines are safe for people aged 12 - 18 years. The probability of re-infection after vaccination was lower among naturally infected populations than in infection-naïve individuals. However, injecting two doses of vaccine (PastoCovac or Sinopharm) in children does not mean complete immunity against re-infection. Considering the potential for future pandemics, particularly those caused by respiratory diseases such as COVID-19, it is imperative that we adhere to preventive protocols.