1. Background
Since the emergence of the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in December 2019 as the causative agent of coronavirus disease 2019 (COVID-19), a global pandemic has unfolded, significantly impacting healthcare systems worldwide (1). According to the World Health Organization (WHO), as of November 8, 2023, there have been 771 820 937 confirmed cases of COVID-19, resulting in 6 978 175 deaths (2). Given the absence of a definitive treatment, achieving herd immunity through vaccination is considered the most effective strategy to reduce the severity of COVID-19, lower mortality rates, and gain long-term control over the pandemic (3, 4).
The WHO reports that several COVID-19 vaccines, utilizing various platforms such as viral vectors, inactivated virus modalities, and messenger ribonucleic acid (mRNA), have received emergency clinical use authorization for preventing SARS-CoV-2 infections (5). The US Food and Drug Administration (FDA) granted the first emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine in December 2020 (6). While COVID-19 vaccines offer significant benefits by reducing the risk of hospitalization and death, it is expected that they may cause different adverse events (7). According to the WHO, recipients of COVID-19 vaccines may experience mild and short-term side effects, including fever, fatigue, headache, muscle aches, chills, diarrhea, and pain or redness at the injection site. Serious or long-lasting side effects of COVID-19 vaccines (such as difficulty breathing, chest pain, confusion, and loss of speech) are rare (8). Most published data from COVID-19 vaccine trials have consistently reported mild-to-moderate side effects, with varying severity depending on the specific type of COVID-19 vaccine (9, 10).
While mild-to-moderate side effects are common with COVID-19 vaccines, ongoing safety monitoring remains crucial to detect adverse events promptly. This monitoring enhances public and healthcare worker confidence, increases vaccine acceptance, and ensures the safe use of these vaccines (8, 11).
2. Objectives
This study aimed to estimate the prevalence of the side effects of 4 COVID-19 vaccine types among vaccinated healthcare workers following the first and second vaccine doses and identify the possible risk factors of COVID-19 vaccine side effects.
3. Methods
3.1. Study Design
This cross-sectional study was conducted among healthcare workers at Afzalipour University Hospital in Kerman, Iran, who had received 2 or more doses of any COVID-19 vaccines available in Iran, including AZD-1222 (Oxford/AstraZeneca), Covaxin, BBV152 (Bharat Biotech), BBIBP-CorV (Sinopharm, China), and Sputnik V (Gamaleya Research Institute). Data collection took place between April 2021 and March 2022, using a structured questionnaire and direct interviews with the study participants. The questionnaire consisted of 4 main sections:
- Demographic information, including age, sex, and underlying medical conditions.
- History of COVID-19 infection and reverse transcription-polymerase chain reaction (RT-PCR) test results.
- Details related to COVID-19 vaccination, including the type of vaccine received, vaccination dates, and the number of doses administered.
- Reporting of possible COVID-19 vaccine side effects experienced after the first and second doses.
The side effects assessed in the questionnaire included:
General symptoms (weakness, fatigue, hypothermia, flu-like symptoms, fever, chills, and dry mouth).
Organ-specific symptoms, including respiratory symptoms (sore throat, runny nose, nasal congestion, hoarseness, cough, shortness of breath, pleuritic chest pain, hemoptysis, and hemothorax), gastrointestinal (GI) symptoms (abdominal pain, nausea, vomiting, diarrhea, loss of appetite, dyspepsia, hiccups, gingivitis, and oral aphthous ulcers), hematological symptoms (thrombocytopenia, vascular thrombosis, and heavy menstrual bleeding), neurological symptoms (drowsiness, confusion, agitation, insomnia, dizziness, headache, vasovagal reactions, double vision, weakness, sensory disturbances, unsteady gait, seizures, and changes in taste and smell), musculoskeletal symptoms (arthritis, joint pain, muscle pain, myositis, back pain, and generalized body aches), cardiovascular symptoms (chest pain, rapid heart rate, high blood pressure, low blood pressure, and cold sweats), renal symptoms (flank pain, difficulty urinating, and kidney dysfunction), skin symptoms (swelling and redness at the injection site, skin rash, anaphylactic reactions, hair loss, and herpes simplex infection), and ophthalmic symptoms (red eyes, conjunctivitis, blepharitis, burning eyes, eye pain, blurred vision, and drooping eyelids).
3.2. Inclusion/Exclusion Criteria
In this study, the inclusion criteria were as follows: Individuals who had received both the first and second doses of 1 of the 4 vaccines (Sputnik V, AstraZeneca, Covaxin, or Sinopharm). Participants were required to specify the type of COVID-19 vaccine they had received, as well as the number of doses. Exclusion criteria encompassed participants who failed to report side effects related to COVID-19 vaccines and those who tested positive for COVID-19 during the study period. Participants were followed up 45 days after each vaccine dose.
3.3. Ethical Considerations
The study protocol received approval from the Local Ethics Committee of Kerman University of Medical Sciences (IR.KMU.AH.REC.1399.181). All participants provided informed consent by signing a consent form. The research was conducted in compliance with relevant guidelines and regulations.
3.4. Statistical Analysis
Statistical analysis was performed using SPSS v. 20 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as frequencies and percentages for demographic variables (age, sex), underlying medical conditions, COVID-19 history, RT-PCR test results, COVID-19 vaccine type, and side effects following the first and second vaccine doses. Categorical variables were compared between different groups using the chi-square or Fisher's exact tests. The level of statistical significance was set at P < 0.05.
4. Results
4.1. Population Characteristics
Sample characteristics of the 861 individuals enrolled in the study are presented in Table 1. Out of the 861 participants who completed our questionnaire, 566 (65.7%) belonged to the 20 - 40 age group, and 566 (65.7%) were females. One hundred and thirty-one (15.2%) had associated comorbid conditions, including diabetes, hypertension, dyslipidemia, cardiovascular, and liver disease, while 268 (31.1%) had a history of SARS-CoV-2 infection before vaccination. Additionally, 338 (39.3%) of the vaccine recipients had a history of positive RT-PCR testing before vaccination. Concerning the type of COVID-19 vaccination, the majority of study participants (38.7%) received the Sputnik vaccine, 32.4% received the AstraZeneca vaccine, 19.6% received the Covaxin vaccine, and 9.3% received the Sinopharm vaccine.
| Characteristics | No. (%) |
|---|---|
| Sex | |
| Male | 295 (34.3) |
| Female | 566 (65.7) |
| Age groups (y) | |
| 20 - 40 | 566 (65.7) |
| 41 - 60 | 272 (31.6) |
| ≥ 61 | 23 (2.7) |
| Underlying disease | |
| Yes | 131 (15.2) |
| No | 730 (84.8) |
| Previous COVID-19 | |
| Yes | 268 (31.1) |
| No | 593 (68.9) |
| RT-PCR test history | |
| Positive | 338 (39.3) |
| Negative | 523 (60.7) |
| Type of COVID-19 vaccine | |
| Sinopharm | 80 (9.3) |
| AstraZeneca | 279 (32.4) |
| Covaxin | 169 (19.6) |
| Sputnik V | 333 (38.7) |
Abbreviations: COVID-19, coronavirus disease 2019; RT-PCR, reverse transcription-polymerase chain reaction.
4.1.1. COVID-19 Vaccine Side Effects Based on Different Variables
The prevalence and frequency of side effects based on the number of doses and type of vaccine are described in Tables 2 and 3. For those who received the Sinopharm vaccine, the most common symptoms after the first and second doses were injection site reactions (21.2%) and general symptoms (17.5%), respectively. Regarding the AstraZeneca vaccine, the most common symptoms were fever and chills (58.8%) after the first dose and general symptoms (29%) after the second dose. In the case of the Covaxin vaccine, general symptoms (32.5%) and injection site reactions (19.5%) were the most common symptoms after the first and second doses, respectively. Among those who received both doses of the Sputnik vaccine, general symptoms were the most common (46.8% after the first dose and 36% after the second dose).
| Symptoms | The First Dose, No. (%) | Second Dose, No. (%) | P-Value a |
|---|---|---|---|
| Injection site reaction | 238 (27.6) | 176 (20.4) | 0.001 |
| Fever and chills | 317 (36.8) | 164 (19) | 0.001 |
| Gastrointestinal symptoms | 65 (7.5) | 35 (4.1) | 0.002 |
| Musculoskeletal symptoms | 56 (6.5) | 44 (5.1) | 0.207 |
| Respiratory symptoms | 23 (2.7) | 13 (1.5) | 0.089 |
| Vascular symptoms | 5 (0.6) | 2 (0.2) | 0.253 |
| Neurological symptoms | 175 (20.3) | 88 (10.2) | 0.001 |
| Ocular symptoms | 10 (1.2) | 5 (0.6) | 0.19 |
| Skin symptoms | 6 (0.7) | 2 (0.2) | 0.15 |
| General symptoms | 368 (42.7) | 264 (30.7) | 0.001 |
| Lymphatic adenopathy | 5 (0.6) | 5 (0.6) | 1 |
a Based on the chi-square test.
| Symptoms | The First Dose, No. (%) | Second Dose, No. (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sinopharm | AstraZeneca | Covaxin | Sputnik | Total | P-Value a | Sinopharm | AstraZeneca | Covaxin | Sputnik | Total | P-Value a | |
| Injection site reaction | 17 (21.2) | 88 (31.5) | 40 (23.7) | 93 (27.9) | 238 (27.6) | 0.128 | 13 (16.2) | 62 (22.2) | 33 (19.5) | 68 (20.4) | 176 (20.4) | 0.727 |
| Fever and chills | 7 (8.8) | 164 (58.8) | 20 (11.8) | 126 (37.8) | 317 (36.8) | 0.001 | 2 (2.5) | 60 (21.5) | 18 (10.7) | 84 (25.2) | 164 (19) | 0.001 |
| Gastrointestinal symptoms | 0 | 33 (11.8) | 5 (3) | 27 (8.1) | 65 (7.5) | 0.001 | 0 | 14 (5) | 10 (5.9) | 11 (3.3) | 35 (4.1) | 0.11 |
| Musculoskeletal symptoms | 1 (1.2) | 17 (6.1) | 13 (7.7) | 25 (7.5) | 56 (6.5) | 0.199 | 1 (1.2) | 20 (7.2) | 10 (5.9) | 13 (3.9) | 44 (5.1) | 0.106 |
| Respiratory symptoms | 0 | 10 (3.6) | 2 (1.2) | 11 (3.3) | 23 (2.7) | 0.169 | 0 | 3 (1.1) | 1 (0.6) | 9 (2.7) | 13 (1.5) | 0.133 |
| Vascular symptoms | 0 | 2 (0.7) | 0 | 3 (0.9) | 5 (0.6) | 0.545 | 0 | 1 (0.4) | 0 | 1 (0.3) | 2 (0.2) | 0.84 |
| Neurological symptoms | 6 (7.5) | 77 (27.6) | 24 (14.2) | 68 (20.4) | 175 (20.3) | 0.001 | 2 (2.5) | 32 (11.5) | 17 (10.1) | 37 (11.1) | 88 (10.2) | 0.113 |
| Ocular symptoms | 0 | 2 (0.7) | 3 (1.8) | 5 (1.5) | 10 (1.2) | 0.511 | 0 | 0 | 2 (1.2) | 3 (0.9) | 5 (0.6) | 0.29 |
| Skin symptoms | 0 | 0 | 2 (1.2) | 4 (1.2) | 6 (0.7) | 0.229 | 0 | 1 (0.4) | 1 (0.6) | 0 | 2 (0.2) | 0.55 |
| General symptoms | 11 (13.8) | 46 (52.3) | 55 (32.5) | 156 (46.8) | 368 (42.7) | 0.001 | 14 (17.5) | 81 (29) | 49 (29) | 120 (36) | 264 (30.7) | 0.009 |
| Lymphatic adenopathy | 0 | 1 (0.4) | 1 (0.6) | 3 (0.9) | 5 (0.6) | 0.73 | 0 | 0 | 1 (0.6) | 4 (1.2) | 5 (0.6) | 0.22 |
a Based on the chi-square test.
In summary, after the first dose of vaccination, the highest and lowest reports of fever and chills, neurological symptoms, and GI symptoms were significantly observed in the AstraZeneca and Sinopharm groups, respectively (P < 0.05, Table 3). After the first dose, other symptoms such as injection site reactions, musculoskeletal symptoms, respiratory symptoms, vascular symptoms, ocular symptoms, skin symptoms, and lymphatic adenopathy were lower in the Sinopharm group but not significantly different. After the second dose, the highest and lowest frequencies of fever, chills, and general symptoms were significantly reported among those who received the Sputnik and Sinopharm vaccines, respectively (P < 0.05, Table 3).
Generally, the most common side effects of vaccines after the first and second doses were general symptoms, fever and chills, injection site reactions, neurological symptoms, and GI symptoms (Table 2). Moreover, after the second dose, the frequency of symptoms, including injection site reactions, fever and chills, GI symptoms, neurological symptoms, and general symptoms, significantly reduced (P = 0.001, Table 2). The frequency of other symptoms after the second vaccination dose also decreased but not significantly (P > 0.05, Table 2).
As shown in Table 4, injection site reactions (P = 0.011), fever and chills (P = 0.001), GI symptoms (P = 0.024), neurological symptoms (P = 0.012), and ocular symptoms (P = 0.022) after receiving the first dose, as well as general symptoms after receiving the first (P = 0.001) and second dose (P = 0.007), were significantly more common among females than males.
| Symptoms | The First Dose, No. (%) | Second Dose, No. (%) | ||||
|---|---|---|---|---|---|---|
| Female (n = 566) | Male (n = 295) | P-Value a | Female (n = 566) | Male (n = 295) | P-Value a | |
| Injection site reaction | 73 (12.8) | 65 (22) | 0.011 | 116 (20.5) | 60 (20.3) | 0.768 |
| Fever and chills | 230 (40.7) | 86 (29.2) | 0.001 | 119 (21.1) | 44 (14.9) | 0.069 |
| Gastrointestinal symptoms | 51 (9) | 14 (4.7) | 0.024 | 27 (4.8) | 8 (2.7) | 0.145 |
| Musculoskeletal symptoms | 42 (7.4) | 14 (4.7) | 0.129 | 31 (5.5) | 13 (4.4) | 0.495 |
| Respiratory symptoms | 18 (3.2) | 5 (1.7) | 0.198 | 11 (1.9) | 2 (0.7) | 0.269 |
| Vascular symptoms | 3 (0.5) | 2 (0.7) | 0.788 | 2 (0.4) | 0 | 0.306 |
| Neurological symptoms | 129 (22.8) | 46 (15.6) | 0.012 | 60 (10.6) | 28 (9.5) | 0.604 |
| Ocular symptoms | 10 (1.8) | 0 | 0.022 | 5 (0.9) | 0 | 0.105 |
| Skin symptoms | 3 (0.5) | 3 (1) | 0.416 | 1 (0.2) | 1 (0.3) | 0.64 |
| General symptoms | 272 (48.1) | 95 (32.2) | 0.001 | 190 (33.6) | 73 (24.7) | 0.007 |
| Lymphatic adenopathy | 5 (0.9) | 0 | 0.105 | 4 (0.7) | 1 (0.3) | 0.499 |
a Based on the chi-square test.
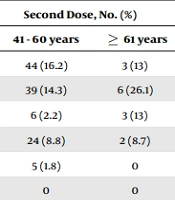
Table 5 presents the status of side effects based on age. After receiving the first dose, fever and chills (P = 0.001), musculoskeletal symptoms (P = 0.018), respiratory symptoms (P = 0.032), and general symptoms (P = 0.044) were significantly more prevalent in the age group 20 - 40 years old. Moreover, in the age group 41 - 60, musculoskeletal symptoms were significantly more common (P = 0.002). After receiving the second dose, GI symptoms (P = 0.023) were significantly more common in the age group 20 - 40 years old, and skin symptoms (P = 0.001) were more common in the age group ≥ 61 years old compared to other age groups (Table 4).
| Symptoms | The First Dose, No. (%) | Second Dose, No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 20 - 40 Years | 41 - 60 Years | ≥ 61 Years | P-Value a | 20 - 40 Years | 41 - 60 Years | ≥ 61 Years | P-Value a | |
| Injection site reaction | 165 (29.2) | 63 (23.2) | 10 (43.5) | 0.093 | 129 (22.8) | 44 (16.2) | 3 (13) | 0.099 |
| Fever and chills | 232 (41) | 70 (25.7) | 15 (65.2) | 0.001 | 119 (21) | 39 (14.3) | 6 (26.1) | 0.155 |
| Gastrointestinal symptoms | 48 (8.5) | 15 (5.5) | 2 (8.7) | 0.307 | 26 (4.6) | 6 (2.2) | 3 (13) | 0.023 |
| Musculoskeletal symptoms | 30 (5.3) | 26 (9.6) | 0 | 0.018 | 18 (3.2) | 24 (8.8) | 2 (8.7) | 0.002 |
| Respiratory symptoms | 21 (3.7) | 2 (0.7) | 0 | 0.032 | 8 (1.4) | 5 (1.8) | 0 | 0.599 |
| Vascular symptoms | 3 (0.5) | 2 (0.7) | 0 | 0.873 | 2 (0.4) | 0 | 0 | 0.593 |
| Neurological symptoms | 118 (20.8) | 50 (18.4) | 7 (30.4) | 0.336 | 56 (9.9) | 30 (11) | 2 (8.7) | 0.853 |
| Ocular symptoms | 5 (0.9) | 5 (1.8) | 0 | 0.420 | 2 (0.4) | 3 (1.1) | 0 | 0.382 |
| Skin symptoms | 3 (0.5) | 3 (1.1) | 0 | 0.595 | 1 (0.2) | 0 | 1 (4.3) | 0.001 |
| General symptoms | 256 (45.2) | 100 (36.8) | 12 (52.2) | 0.044 | 174 (30.7) | 84 (30.9) | 6 (26.1) | 0.889 |
| Lymphatic adenopathy | 1 (0.2) | 4 (1.5) | 0 | 0.065 | 4 (0.7) | 1 (0.4) | 0 | 0.777 |
a Based on the chi-square test.
Results in Table 6 show that after receiving the first and second doses of vaccination, musculoskeletal and neurological symptoms were significantly more common in patients with underlying diseases than in those without underlying conditions (P < 0.05).
| Symptoms | The First Dose, No. (%) | Second Dose, No. (%) | ||||
|---|---|---|---|---|---|---|
| Yes (n = 131) | No (n = 730) | P-Value a | Yes (n = 131) | No (n = 730) | P-Value a | |
| Injection site reaction | 46 (35.1) | 92 (12.6) | 0.093 | 28 (21.4) | 48 (6.5) | 0.789 |
| Fever and chills | 45 (34.4) | 272 (37.3) | 0.525 | 22 (16.8) | 142 (19.5) | 0.049 |
| Gastrointestinal symptoms | 9 (6.9) | 56 (7.7) | 0.749 | 5 (3.8) | 30 (4.1) | 0.876 |
| Musculoskeletal symptoms | 14 (10.7) | 42 (5.8) | 0.035 | 12 (9.2) | 32 (4.4) | 0.022 |
| Respiratory symptoms | 4 (3.1) | 19 (2.6) | 0.768 | 3 (2.3) | 10 (1.4) | 0.044 |
| Vascular symptoms | 0 | 5 (0.7) | 0.342 | 0 | 2 (0.3) | 0.549 |
| Neurological symptoms | 37 (28.2) | 138 (18.9) | 0.014 | 20 (15.3) | 68 (9.3) | 0.038 |
| Ocular symptoms | 2 (1.5) | 8 (1.1) | 0.67 | 2 (1.5) | 3 (0.4) | 0.122 |
| Skin symptoms | 1 (0.8) | 5 (0.7) | 0.921 | 0 | 2 (0.3) | 0.54 |
| General symptoms | 62 (47.3) | 306 (41.9) | 0.249 | 55 (42) | 209 (28.6) | 0.002 |
| Lymphatic adenopathy | 1 (0.8) | 4 (0.5) | 0.765 | 1 (0.8) | 4 (0.5) | 0.765 |
a Based on the chi-square test.
There was no significant difference between the group with a history of COVID-19 and the group without a history regarding the prevalence and frequency of complications after the first and/or second dose. However, neurological symptoms (P = 0.033), general symptoms (P = 0.001), and skin symptoms (P = 0.04) after receiving the first dose of the vaccine were significantly more common in the group with a history of positive RT-PCR tests. Additionally, injection site reactions after receiving the second dose were significantly more common in individuals with a positive RT-PCR test history (P = 0.006).
5. Discussion
This survey-based study assessed the side effects of the first and second doses of four vaccines mainly used in a university hospital in Iran. Our study revealed several side effects following COVID-19 vaccination, primarily including general symptoms, fever and chills, injection site reactions, neurological symptoms, and GI symptoms, especially after receiving the first dose. The frequency of individuals experiencing such symptoms after the first vaccine dose was significantly higher than those experiencing them after the second one.
Recent reports on COVID-19 vaccine side effects have been summarized in Table 7. This finding is consistent with existing studies, which report that side effects after the first vaccine dose were more frequent than after the second dose. In a study conducted among the Jordanian population who received the AstraZeneca Vaxzevria, Pfizer-BioNTech, and Sinopharm vaccines (12), researchers asserted that, except for symptoms such as chills, sexual disturbance and lymph node enlargement, others were more frequent after the first vaccine dose than after the second one. In studies by Babaee et al. (13) and Logunov et al. (14), fewer adverse effects were reported after the second doses of the Sinopharm and AstraZeneca vaccines. However, the Sputnik V vaccine had the opposite pattern in that the second dose caused more adverse reactions. On the contrary, many findings from Pfizer and AstraZeneca vaccine studies showed that the prevalence of local and systemic side effects was higher among those who received the second dose than the first one (15-18). The immune system's ability, which functions more actively after the first dose of AstraZeneca, may explain the severity of side effects in this phase (19).
| Study Authors | Year/Country | Vaccine Type | Population Age | Population Sex; No. (%) | Clinical Presentation | Interval Between Vaccine and Side Effects | Outcome |
|---|---|---|---|---|---|---|---|
| Yadegarynia et al. (20) | 2023/Iran | Oxford-AstraZeneca; Sinopharm; Sputnik V | Healthcare workers age range: 18 - 70 years | Female: 257 (68.2); male: 120 (31.8) | The most common side effects after the first dose compared to the second dose were local reactions at the injection site (26.5% vs. 14.3%), systemic side effects (52% vs. 31.8%), flu-like symptoms (78.5% vs. 42.7%), and headache (24.7% vs. 13%). | Not specified | Healthcare workers mostly experienced flu-like symptoms and local reactions at the injection site after vaccination, particularly following the first dose. Side effects were more pronounced with the AstraZeneca and Sputnik V vaccines compared to Sinopharm. |
| Omeish et al. (12) | 2022/Jordan | Oxford-AstraZeneca; Pfizer-BioNTech; Sinopharm | 18 years or older | Female: 684 (63); male: 402 (37) | The majority of participants (89.9%) reported side effects after receiving the first vaccine dose, including pain at the injection site (78.4%), fatigue (51.8%), myalgia (37.6%), headache (33.1%), and chills (32.3%). Gastrointestinal side effects occurred less frequently. | Immediately; first day; first week; second week; third week | The AstraZeneca vaccine exhibited a higher frequency of side effects compared to the Pfizer vaccine, while the Sinopharm vaccine demonstrated the lowest incidence of side effects. |
| Babaee et al. (13) | 2022/Iran | Oxford-AstraZeneca; Sinopharm; Sputnik V | 18 years or older | Female: 2 648 (55.46); male: 2 127 (44.54) | The most common adverse effects after the first dose included fatigue (28.37%), chill/fever (26.86%), and skeletal pain (22.38%). These adverse effects persisted for the second dose, though their prevalence was lower. | The first 72 h after receiving both doses | The Sputnik V vaccine has the highest rate of adverse effects, closely followed by AstraZeneca and Sinopharm vaccines. |
| Riad et al. (17) | 2021/Czech Republic | Pfizer-BioNTech | Healthcare workers Age ranged between 19 and 78 years old. | Female: 776 (88.5); male: 100 (11.4) | The most common side effects were injection site pain (89.8%), fatigue (62.2%), headache (45.6%), muscle pain (37.1%), and chills (33.9%). | Oral side effects' onset was: Within 1 - 3 days the first week; the second week; the third week; the fourth week. | The distribution of side effects closely matched the manufacturer's data, particularly regarding their correlation with the younger age group and the second dose. |
| Mousavi et al. (19) | 2021/Iran | Oxford-AstraZeneca; Sinopharm; Sputnik V | Healthcare workers 18 years and older | AstraZeneca: Female: (41.7); male: (58.3); Sputnik V: Female: (55.5); male: (44.5); Sinopharm: Female: (52.3); male: (47.7) | Fever, myalgia, and pain were the most common side effects across all three types of vaccines. | The next 30 days after receiving each dose | Post-vaccination adverse effects were mild in all groups and managed with analgesics. The AstraZeneca group experienced more intense systemic reactions, which were more frequent after the first dose compared to other groups. |
| Lippi et al. (21) | 2021/Italy | AstraZeneca Vaxzevria; Moderna; Pfizer Comirnaty | The database of the Italian Medicines agency contains voluntarily reported adverse drug reactions. | Not specified | The most common mild side effects were: Fever (13.2%), headache (10.3%), myalgia (8.7%), pain at the injection site (8.3%), arthralgia (7.9%), fatigue (6.2%). | After receiving a dose of 1 of the 3 vaccines | After receiving the Moderna COVID-19 Vaccine, pain at the injection site, lymphadenopathy, and malaise was more common compared to the AstraZeneca Vaxzevria vaccine, while headache, myalgia, arthralgia, fatigue, and chills were less frequent (all P < 0.001). |
| Al Bahrani et al. (22) | 2021/Saudi Arabia | ChAdOx1 (AZD1222) | 18 years or older (range 19 - 83) years | Female: 302 (19); male: 1 290 (81) | The most common symptoms were pain at the site of injection (30.5%), musculoskeletal symptoms (27.5%), skin rash (19.2%), gastrointestinal symptoms (23.8%) and fever (31.3%). | 7 and 21 days | No major side effects or breakthrough infections were observed during the observation period for the ChAdOx1-S vaccine. Reported symptoms varied between males and females and across different age groups. |
| Abu-Hammad et al. (23) | 2021/Jordan | AstraZeneca Vaxzevria; Pfizer-BioNTech; Sinopharm | Healthcare workers age group: ≤ 45 years; > 45 years | Female: 289 (70.7); male: 120 (29.3) | The most common side effects were injection site (74%), fatigue (52%), myalgia (44%), headache (42%), and fever (35%) mainly after the first dose. | Not specified | COVID-19 vaccine side effects in Jordan fall within the expected common range for these vaccines. |
| Kamal et al. (24) | 2021/India | ChAdOx1 nCoV-19 Covaxin | Healthcare workers age > 18 years | Female: 224 (22.8); male: 757 (77.2) | The most common side effects after the first dose were feeling unwell (20.6%), headache (18.4%), fever (13.04%), and fatigue (14.27%). The most common side effects after the second dose were feeling unwell (8.1%), headache (5.7%), muscle aches (4.4%), and decreased appetite (4.9%). | 48 hours, day 8, 15, 22, and 28 after the first and second doses of the vaccine | Short-term adverse events were mainly observed within the first 48 hours, with the incidence decreasing in subsequent weeks and no occurrences after 15 days at both doses. |
| Zare et al. (25) | 2021/Iran | AZD1222; Covaxin; Sputnik V | Healthcare workers age range: 20 - 67 years | Female: 287 (57); male: 216 (43) | The most common side effects in all three vaccines were injection site pain (62.1%), fatigue (43.9%), muscle pain (42.5%), and fever (40.6%) | One week | Injection site pain was the main common side effect across the three vaccine groups, with age and sex being significant factors in the prevalence of side effects. |
| Menni et al. (26) | 2021/UK | AstraZeneca Vaxzevria; Pfizer-BioNTech | General population (app users) age (≤ 55 years vs > 55 years) | BNT162b2: Female: 193 506 (62.3); male: 116 804 (37.7); ChAdOx1 nCoV-19: Female: 199 269 (57.7); male: 146 011 (42.3) | The most common systemic side effects reported were fatigue and headache, whereas tenderness and local pain around the injection site were the most frequently reported local side effects. | 8 days | Systemic and local side effects after vaccination occur at lower frequencies compared to phase 3 trial reports, while both vaccines reduce the risk of SARS-CoV-2 infection after 12 days. |
| Babamahmoodi et al. (27) | 2021/Iran | Sputnik V | Healthcare workers age range: 19 - 78 years | Female: 1 982 (61.2); male: 1 254 (38.8) | The most common side effects were pain in the injection site (56.9%), fatigue (50.9%), body pain (43.9%), headache (35.7%), fever (32.9%), joint pain (30.3%), chilling (29.8%) and drowsiness (20.3%). | 12 hours: 12 - 24 hours; 24 - 48 hours; > 48 h-7 day | The manufacturer's reports on the vaccine's high humoral immunogenicity against COVID-19 were confirmed despite a higher overall rate of adverse effects compared to interim trial results. |
It is essential to mention that fever and chills were among the most frequently reported symptoms. However, the most frequent reports of fever and chills in our study after receiving the first and second doses were significantly observed in the case of AstraZeneca (P < 0.05).
A similar finding from the phase 3 clinical trials confirmed that the highest frequency of fever, up to 24%, was associated with the AstraZeneca vaccine (21). In another study from Saudi Arabia, the most common symptoms associated with the ChAdOx1 (AZD-1222) vaccine in 1592 vaccinated volunteers after the first dose were fever (498, 31.3%), injection site pain (485, 30.5%), musculoskeletal symptoms (438, 27.5%), GI symptoms (379, 23.8%), and skin rash (307, 19.2%), which are consistent with our results after the first dose (22).
In this study, symptoms including fever, chills, neurological, and GI symptoms had significantly lower frequencies in the case of Sinopharm after receiving the first dose. Additionally, the lowest frequencies of symptoms, such as fever, chills, and general symptoms, were significantly observed in the Sinopharm group after receiving the second dose. In randomized, double-blind, placebo-controlled phase 1 and 2 trials in China, mild adverse reactions, including fever (6%), fatigue (3%), headache (1%), and myalgia and joint pain (1%), were observed in the case of the Sinopharm vaccine (28). Similar to our findings, a review conducted by Wu et al. (29) showed that the rates of local and systemic reactions in the case of inactivated vaccines such as Sinopharm were significantly lower than those for Pfizer and AstraZeneca vaccines. In a study by Abu-Hammad et al. (23), Sinopharm is indicated as a “quiet” vaccine since it was significantly associated with rare symptoms.
The Sputnik V vaccine (rAd26-S and rAD5-S), with a safe and tolerable profile based on phase 1/2 studies, strengthens both humoral and cell-mediated immunity, resulting in efficient immune responses (30). Clinical trial studies of Sputnik V have shown that the most common side effects are injection site pain, fever, headache, fatigue, and muscle and joint pain (31). In the present study, side effects in the Sputnik V group were more noticeable after the second dose. Two cases with supraclavicular lymphadenopathy were observed following the administration of the second dose of the Sputnik V vaccine. One-week and 2-week follow-up ultrasonography showed a decreased size of the nodes, followed by resolution within 1 month. However, the frequency of only fever, chills, and general symptoms was significant, and most side effects showed no significant association with the number of doses. This trend was also observed in Omeish et al. (12), who reported that fever and chills were significantly higher in the Sputnik group after the second dose.
Regarding Covaxin, data are inadequate. The most common side effects of the Covaxin vaccine in our study were general symptoms (61.6%), injection site reaction (43.2%), neurological symptoms (24.3%), fever and chills (22.5%), and musculoskeletal symptoms (13.6%), while in the study performed by Ella et al., the side effects reported for all were < 10% (32). In another report by Kamal et al., the most prevalent symptoms were headache (17.4%), fever (12.5%), fatigue (12.3%), and muscle pain (11.2%) (24). In contrast to our study, the rate of side effects in both studies was lower, while in Zare et al.'s study, injection site pain (83.7%) and muscle pain (20%) (25) were more common than in our study. These discrepancies may be due to differences in research participants or data collection procedures.
Some research findings suggest that younger people experience more side effects after vaccination than older individuals (29, 33). In the present study, the frequency of side effects was higher in the age group of 20 - 40 years old. Although this difference was not significant for all complications, fever and chills, musculoskeletal symptoms, respiratory symptoms, and general symptoms were significantly more common in the 20-40-year-old age group than in the other groups after receiving the first dose. Additionally, the 20 - 40 age group experienced more GI symptoms after receiving the second dose than the other age groups.
Sex differences in COVID-19 vaccine side effects have also been observed in many studies (22, 25, 26, 31). According to studies conducted by Zare et al. (25) and Al Bahrani et al. (22), females were more likely to report injection site pain, while men were more likely to report skin rash (81.1%) and fever (76.9%). Similarly, in the present study, females significantly experienced injection site reactions (P = 0.011, 30.6%) after receiving the first dose than males. In our study, fever and chills (40.7%), GI symptoms (9%), neurological symptoms (22.8%), and ocular symptoms (1.8%) after receiving the first dose, as well as general symptoms after the first (48.1%) and second dose (33.6%), were reported more frequently in females than in males. Individual differences in pain tolerance thresholds may explain the difference in the prevalence of injection site pain between males and females (25).
This study examined the prevalence of post-vaccination side effects following the first and second doses of prevalent COVID-19 vaccines in a university hospital in Iran. Our findings align with the existing literature, displaying a trend of increased side effects after the initial vaccine dose compared to subsequent doses.
Our observations resonate with previous studies comparing side effects after the first and second doses. Babamahmoodi et al. conducted a study among healthcare workers in Iran, focusing on Sputnik V vaccine administration, reporting fewer adverse reactions after the second dose of Sputnik V (27). Similarly, Yadegarynia et al.'s research on healthcare workers in Tehran noted reduced side effects in the second dose compared to the first (20).
Consistent with our findings, the study of Babamahmoodi et al. identified a trend of lower adverse reactions after the second Sputnik V dose (27). In contrast, Yadegarynia et al. also reported a decline in side effects following the second dose across various COVID-19 vaccines among healthcare workers (20).
Moreover, our study echoed the frequent occurrence of fever and chills after AstraZeneca vaccinations, consistent with Yadegarynia et al.'s findings in healthcare workers in Tehran (20). These consistent observations reinforce the understanding of vaccine-specific side effect profiles and the variability in their prevalence between doses.
The present study also highlighted lower frequencies of side effects, including fever, chills, and neurological and GI symptoms, particularly after the first dose of the Sinopharm vaccine. This aligns with Babamahmoodi et al.'s study on healthcare workers in Iran, further emphasizing reduced side effects with Sinopharm vaccination (27).
Additionally, sex disparities in side effects were observed, with females reporting higher occurrences, which is consistent with Yadegarynia et al.'s findings (20). Furthermore, our study, similar to Babamahmoodi et al.'s research, highlighted the relationship between underlying diseases and increased musculoskeletal and neurological symptoms post-vaccination (27).
Notably, our study has limitations, including incomplete responses to inquiries and a smaller sample size for the Sinopharm vaccine, which may have influenced our observations. Future research with robust methodologies and larger sample sizes is warranted to comprehensively understand side effect variations across different vaccines, doses, and demographic groups.
In conclusion, our observations, supported by findings from studies by Babamahmoodi et al. (27) and Yadegarynia et al. (20), underscore the prevalence of post-vaccination side effects between the first and second doses of COVID-19 vaccines. These collective insights emphasize the need for continued surveillance and comprehensive assessment to delineate vaccine-specific side effect patterns and optimize vaccination strategies.
Moreover, the prevalence of musculoskeletal and neurological symptoms after receiving the first and second vaccination doses was significantly higher in participants with underlying diseases than in those without underlying conditions. The possibility of individuals with underlying diseases experiencing pain at a lower threshold compared to those without such conditions may explain this finding (29).
In summary, it is essential to note that no severe side effects were detected in any of the participants, and all side effects are short-term. However, this study has some limitations, including incomplete inquiry responses, potential bias in reporting side effects, a short follow-up period, and a small sample size for the Sinopharm vaccine. Activating a well-governmental follow-up system and examining side effects in a larger population, as well as assessing side effects in different vaccines with longer follow-ups, can provide valuable insights for future research.
The most widespread side effects observed for the four COVID-19 vaccines, Sputnik V, AstraZeneca, Covaxin, and Sinopharm, were general symptoms, fever and chills, injection site reactions, neurological symptoms, and GI symptoms. The prevalence of these side effects was generally higher in females than in males. Additionally, the side effects were age-related, with older adults experiencing fewer side effects after the first vaccine dose than younger adults. Overall, the reported symptoms, including general symptoms, fever and chills, injection site reactions, neurological symptoms, and GI symptoms, were significantly more common after receiving the first vaccine dose compared to the second dose. However, further research is needed to evaluate the long-term symptoms and safety profiles of COVID-19 vaccines.