1. Introduction
Herpes simplex virus encephalitis (HSVE) is one of the most common reasons for necrotizing encephalitis in the brain (1). HSV type 1 is commonly responsible for adult encephalitis, while HSV type 2 is responsible for newborn encephalitis. Altered consciousness or altered behavior, followed by seizure and headache is reported as the most common form of presentation in cases of acute encephalitis (2-4). Diagnosis of herpes zoster is primarily based on the history and physical findings. Polymerase chain reaction (PCR) testing of vesicular fluid, a corneal lesion, or blood is the gold standard to detect the virus (5). Treatment of this disease in the early stage with acyclovir affects mortality (3, 6). Since the common minor bleedings during HSVE are rarely identified, ischemic stroke rarely occurs (7, 8). Ramsay-Hunt syndrome (RHS) is characterized by the re-activation of the latent varicella zoster virus (VZV) in the geniculate ganglion of the facial nerve and facial nerve lesion secondary to outer ear way herpetic infection and presents with peripheral facial nerve paralysis in the clinic (9-11). Encephalitis, a neurological complication of VZV, which is a member of herpes virus family, can occur in the form of large or small vascular arteritis (12-14). Vasculopathy secondarily developed after HSV infections is responsible for the occurrence of this stroke situation (15). Rare cases as vasculopathy and ischemic stroke after RHS are described in the literature (16). Antiphospholipid syndrome (APS) is the most common reason for acquired thrombophilia leading to venous and arterial thrombosis (17).
In the current study, a case admitted to our clinic with the situation of stroke considered to be developed as secondary to organized hematoma oppression following HSVE and a patient that developed anterior cerebral artery (ACA) and middle cerebral artery (MCA) territory infarction after RHS are discussed.
2. Case Presentation
2.1. Case 1
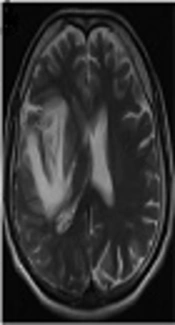
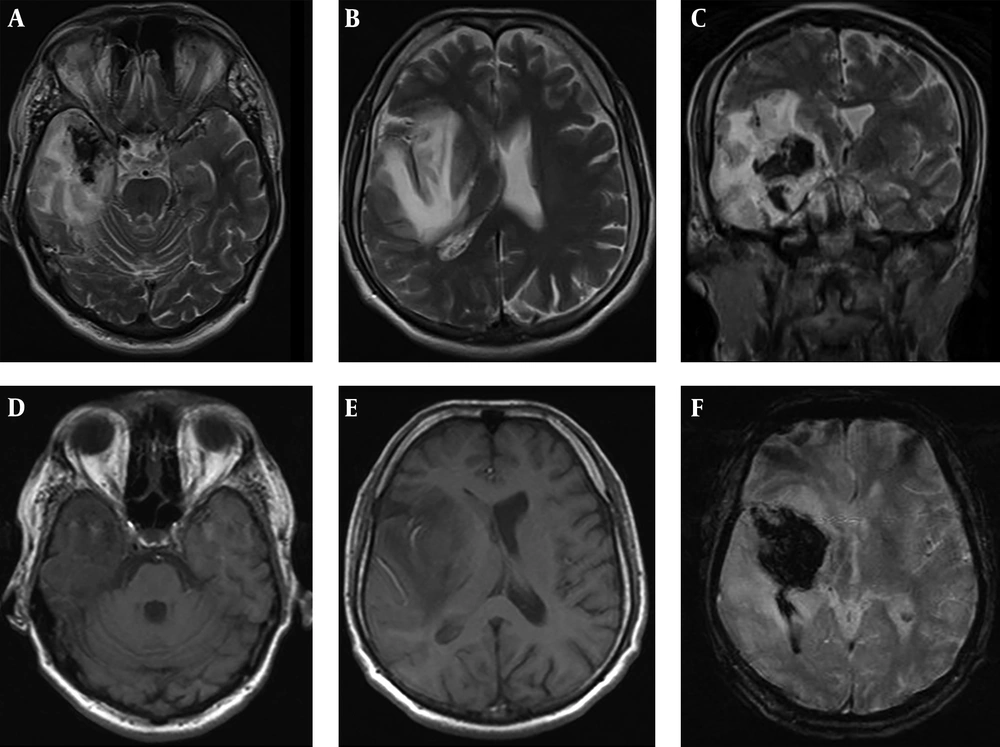
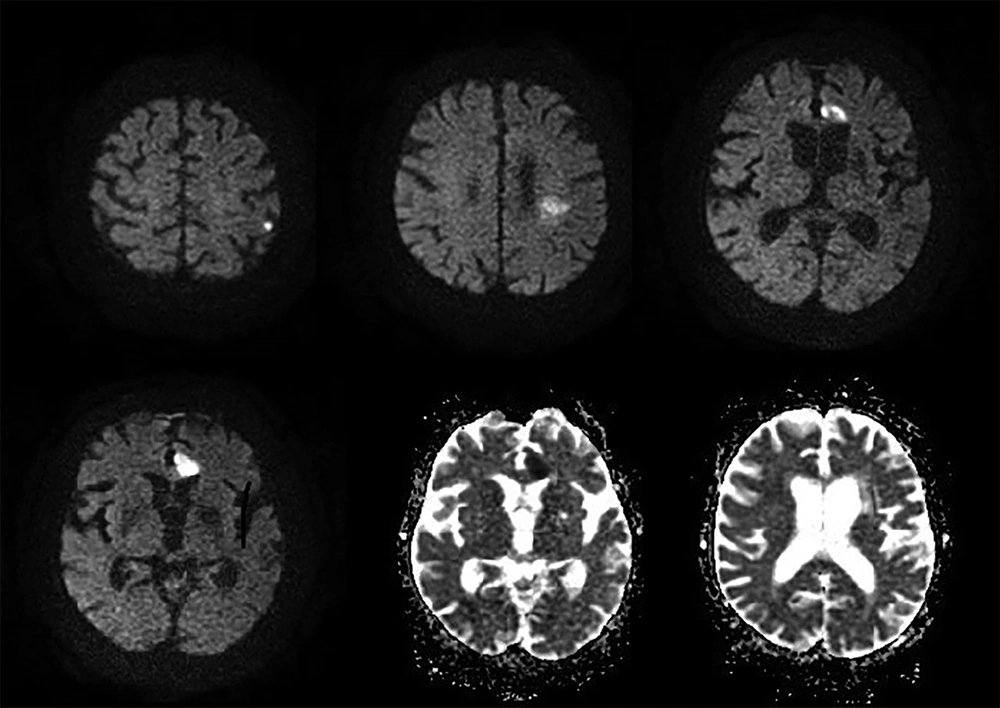
A 41-year-old male patient, working as a driver, was admitted to the emergency service with the presentation of right MCA infarction. Patient’s history revealed that he was first admitted to the infectious diseases department of a polyclinic due to headache, changes in personality, and epileptic seizure and was followed up with HSVE diagnosis. In lumbar puncture, HSV type 1 was detected from his spinal fluid with real-time PCR technique. On the 3rd day of the admission to hospital, the magnetic resonance imaging (MRI) of brain was conducted since the patient started to feel weakness in the left arm and left leg. Development of hemorrhagic transformation was observed on the right temporal area (Figure 1). After initiating the antiedema treatment, almost a full recovery on the patient's weakness was observed.
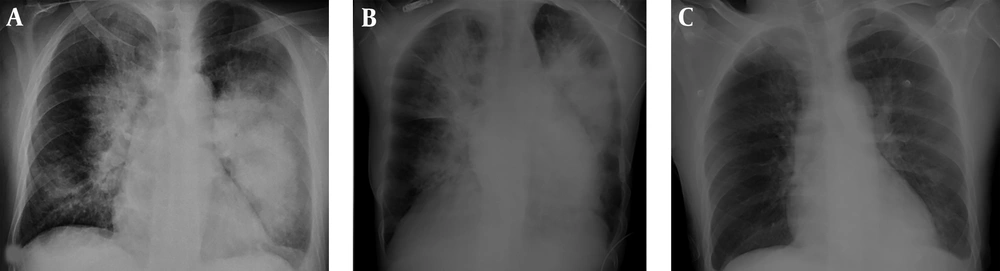
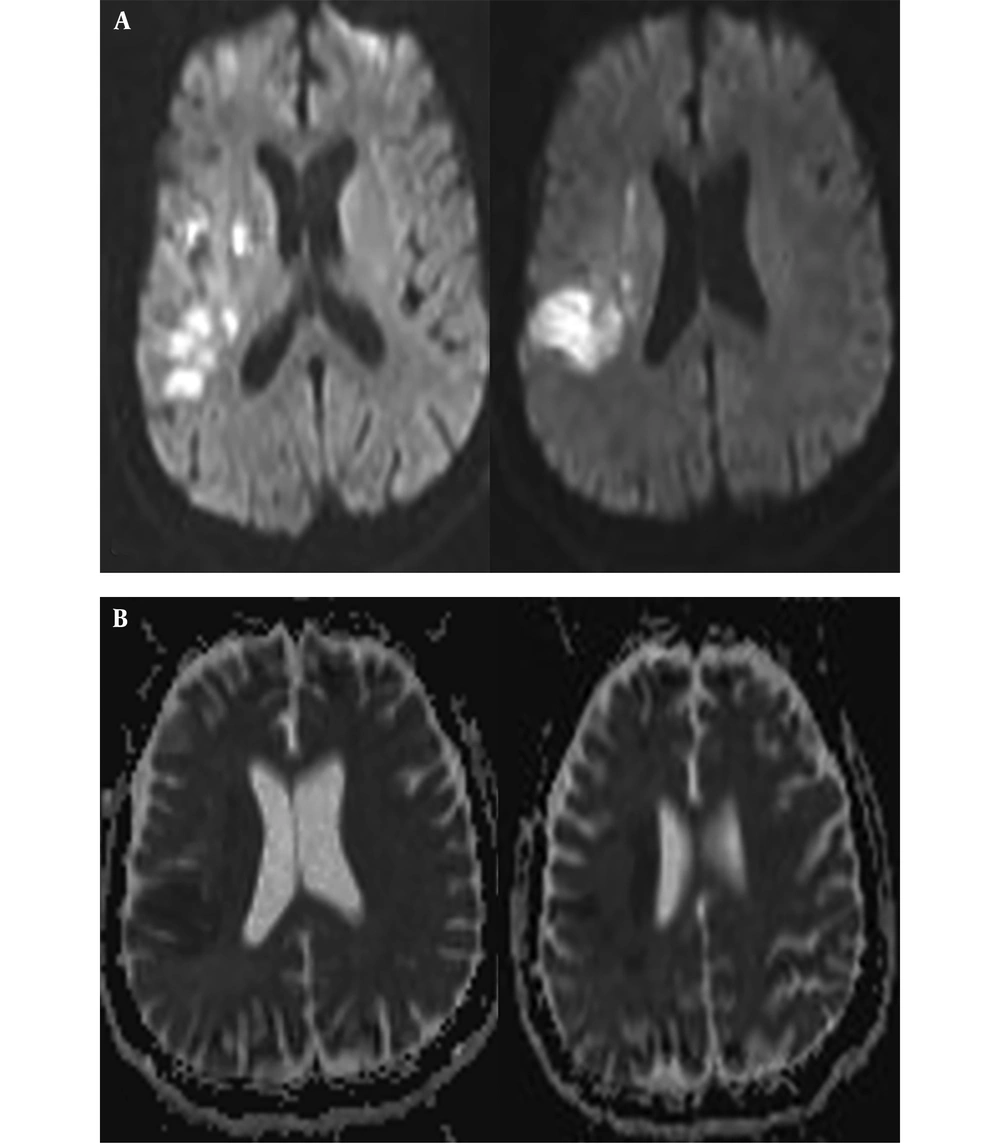
On the 15th day of the treatment, posteroanterior (PA) lung graphy was conducted as the patient developed respiratory failure, and bilateral consolidated areas were observed. Ceftriaxone therapy was replaced with meropenem 3 g/day, linezolid 1200 mg/day, and acyclovir 2250 mg/day, and patient was transferred to the intensive care unit (ICU) as the he was suspected to acute respiratory distress syndrome (ARDS) and pneumonia. Patient completely recovered in 72 hours from dyspnea and respiratory distress. In PA lung graphy, bilateral consolidated areas were not observed (Figure 2). The final inspection of the neurological data revealed no remarkable point in the clinically improved patient. After completing the acyclovir and antibiotic therapy on the 21st day, patient was discharged from the hospital on the 30th day with enoxaparin 0.4 mg/day and carbamazepine 400 mg/day treatment without any sequela; five days after discharge (on the 35th day of the admission) loss of strength developed in the patient. Muscle strength on left leg and left arm were 4/5 during neurological inspection. Hematoma was observed on the right temporal area in the conducted brain BT and coherent diffusion restriction was observed with acute infarct coherent concordant with right middle cerebral artery territory in brain MR diffusion (Figure 3). The patient admitted to neurology department received acetylsalicylic acid 150 mg/day and karbamezapin 400 mg/day. Acute infarct areas coherent with right MCA irrigation area were observed during the conducted brain MR and MR diffusion in the patient. The patient was diagnosed with ischemic stroke developed as a result of organized hematoma oppression on right MCA caused by HSVE. Patient was discharged with normal neurologic inspection, except frust hemiparesis on left leg and left arm. There was no significant pathology in vertebral and carotid Doppler ultrasonography (USG) in terms of thromboembolism. Echocardiography of the patient was normal in terms of cardioembolism. On the examinations required for early ischemic stroke etiology of the patient, antinuclear antibodies (ANA), anti-ds DNA, an extractable nuclear antigen (ENA) profile, anticardiolipin lgG and lgM, and anti-phospholipid lgG and lgM were negative. Lupus anticoagulant test result was 43 seconds (30-37) while F-V Leiden mutation was heterozygote and MTHFRA A 1298 C homozygote mutagen was identified. On the 6th month inspection of the patient, there was no significant pathology on the neurologic examination except minimal paresis on upper left and lower left.
2.2. Case 2
An 87-year-old female was admitted to the ear-nose-throat (ENT) clinic complaining of inability to close the right eye and left contraction in mouth corner. During the examination, vesicular lesions on her right outer ear way were inspected and a diagnosis of Ramsay-Hunt syndrome due to herpes zoster infection was made and she was admitted to ENT clinic. Her initial treatment was planned as acyclovir tablet 5x1 (for one week), prednisolone ampule 80 mg/day (for five days). During the follow-up, it was noted that she had frust right hemiparesis, and brain MR and MR diffusion were taken. After the detection of left ACA-MCA territory infarction, she was admitted to the neurology clinic (Figure 4). On neurological examination, she had right peripheral facial paralysis, her global motor strength was 4/5 on the right upper and lower extremities, her right plantar reflex was extensor. There was no significant pathology in carotid and vertebral USG; there was no detected thrombus on transthoracic echocardiogram (TTE) and her holter rhythm result was in normal limits. Enoxaparin 0.4 mg/day, and acetylsalicylic acid 100 mg/day were added to her current treatment in the neurology clinic. The patient without worsening in her symptoms was discharged from the clinic with physical therapy and rehabilitation policlinic control recommendation. Within the one-month follow-up, it was observed that her ear vesicles completely disappeared, but there was sequela in her right facial paralysis.
3. Discussion
HSVE is the most important reason of fatal encephalitis. Early antiviral treatment is efficient. Necrotic, inflammatory, and hemorrhagic lesions and neuron losses cause common destruction in brain parenchyma and result in death. Most commonly it holds the temporal lobes and necrotic lesions are accompanied by excessive edema. Fever, headache, changes in personality, focal neurologic deficits, and epileptic attacks are the first admission reasons (2). First admission reasons in the 1st patient were also continuous headache and confusion in recent days. Lesion coherent with typical HSV as edema developing and hemorrhagic transforming on right temporal were present on the 1st conducted cranial MRI. Patient was discharged after recovering with minimal sequela after receiving antiviral, and antiepileptic treatment for one month. However, on the 5th day of the discharge, patient was admitted to hospital with acute diffusion restriction, coherent with right MCA area and stroke status. As known, hemorrhagic transformation during HSVE is frequently observed. In the literature, 20 cases were reported with stroke status developed with intracerebral hematoma coexistence (7, 18). Hematoma development on these cases can be frequently observed in the 1st admission or within the 1st two weeks. In pathophysiology, it was claimed that this hematoma was developed as secondary to intracranial hypertension and veinlet tear caused by vasculitis. Even though there was intense bleeding in the patient under study at the beginning of hematoma, stroke occurred 35 days after the incident (19). In the current case, hemorrhagic transformation was present in the cranial MR conducted on the 1st admission to emergency service prior to acyclovir treatment; but in this period, clinical symptoms of stroke were not present in the patient. The current case explains that the stroke status occurred on the later period.
Ischemic strokes can be rarely observed as a result of HSV infection and the reason behind it is still waiting to be explained (8). Another approach reports as HSV causing vasculitis and hematoma can develop as a result of it, while at the same time it may cause ischemic stroke (15, 20). In the literature, it was reported that central nervous system vasculitis developed during HSV infection are presented with blood vessel wall uptake and it might cause both hemorrhagic and ischemic stroke especially in young patients with stroke (20, 21). In the literature it is especially emphasized that preventing vasculitis in HSV infection might be the addition of low-dose steroid treatment (22). In the current case, both patients were not treated with low-dose steroids during HS viridae infection, and consequently, the risk of developing vasculitis was high. In the previously conducted studies, edema was observed twice as the periods where erythrocyte lysis occurred at the end of the 2nd week and new clot retraction occurred within 48 hours of intracranial bleedings. It is emphasized that excess in edema observed in early stage is in direct proportion with the size of the hematoma and negatively affects prognosis (23).
MCA ischemia occurred at the end of 4th week in the 1st case of the current study. It was not observed in the intense oppressive periods of hematoma. Cranial MR angiography was not sufficient to identify the non-occluded veins of MCA in this area. Vasospasm related to hematoma is a complication observed in subarachnoid bleedings and intracerebral hematomas opening to ventricles from seven to fourteen days (24). Vasospasm is reported in MCA branches in early periods for intracranial hemorrhages (25). Contribution of perihematomal ischemia to edema is disputed, flow decrease in this area starts in a very short time and declines within hours. In the current study ischemia in MCA area occurred one month after hematoma in the 1st case. However, ischemia was observed earlier in the 2nd case. Rather than possibility of secondary vasospasm following the hematoma oppression bleeding, it was concluded that it was coherent with early and late complication of vasculitis related with HSVE and hematoma at the beginning in the 1st case. Among the neurological complications of VZV, encephalitis can be as a large artery or small arterial arteritis. MCA and ACA are the most affected large vessels and patients can come with stroke status. In addition, small vessel arteritis is observed as a nonspecific white matter involvement in cranial imaging and is important in the differential diagnosis of demyelinating diseases (12). In accordance with the literature, MCA-ACA involvement in the 2nd patient that had a stroke after VZV infection was compatible with those of large arterial vasculitis (12). Antiphospholipid syndrome is the most common reason for acquired thrombophilia leading to venous and arterial thrombosis (17). Thus, anti-phospholipid and anticardiolipin antibodies in the current study patient were negative, lupus anticoagulant positivity was present and patient was diagnosed with anti-phospholipid syndrome. In the literature, coexistence of anti-phospholipid syndrome and HSVE was diagnosed in young patients with stroke (26). Especially on the cases with coexistent anti-phospholipid syndrome and HSV infection, presence of microtrombus and vascular thrombosis were observed (27).
Especially higher risk of hereditary thromboembolism as per venous thromboembolism and its insignificancy as per arterial stroke were presented in the recently conducted studies (28). In the current study, F-V Leiden heterozygote and MTHFR A 1298 C homozygote mutations were diagnosed, but this hereditary thrombophilia that was not the main responsible factor for arterial stroke was considered as the etiology, especially for acquired thrombophilia and HSV vasculitis stroke.
A prominent incident in the 1st case was the ARDS/pneumonia developed on the 15th day of the treatment and recovered completely within 2 - 3 days. The current study patient was followed up in ICU and ventilator support was provided during this period. ARDS is an acute dyspnea and hypoxemia situation developed within a few days from the incidence serious conditions such as trauma, aspiration, and sepsis. It is also presented as a mortal condition, with long-lasting recovery, developing as secondary to barrier disturbance, with increasing shunt, and vascular and alveolar epithelium damage besides cardiac edema (29). Neurogenic pulmonary edema is the situation of respiratory failure occurring hours after the serious neurogenic incident without other reasons causing lung edema, and is developed by increase in sympathetic activity progressed by increase in alveolar and interstice liquid, which develops on instant intracranial pressure increase, hypothalamic bulbar solitary nucleus, and spinal cord autonomic nucleuses (30). PA lung graphy in the current study patient almost completely treated and the patient clinically recuperated within 48 - 72 hours. in the current studied cases, neurogenic pulmonary edema was considered in forefront. For the patients with immediate development of hypoxia and lung infiltration findings, recovery was observed within 48 - 72 hours, therefore, neurologic pulmonary edema should be considered as a distinguishing factor.
As a result, possibility of vasculitis process was responsible for both of the studied cases. There were early vasculitis complications in the current study 2nd case, which developed in the late stage in the 1st case of the current study. Especially on the patients diagnosed with anti-phospholipid syndrome and patients with hereditary vasculitis, HSVE makes it easier to develop vasculopathy development and makes the clinical situation more dramatic. Also, neurogenic pulmonary edema should be considered for the cases of immediate hypoxia and pulmonary edema occurred following a neurologic incident observed in the current study 1st patient.
Using steroids at the very beginning of HSVE seemed to have positive effect; here, using steroid after hematoma was important as it prevented the development of the last complication.