1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for COVID-19, which first emerged in Wuhan, China, in 2019. Given the role of vaccination as the most effective strategy to control the pandemic, urgent attention was directed to the spike protein on the SARS-CoV-2 surface as a suitable target for vaccine development (1-3). Overall, 140 vaccines are undergoing clinical development, including DNA, mRNA, subunit, and vector vaccines, some of which have been approved for human use (4).
In the COVID-19 era, patients with multiple sclerosis (PwMS) represented a population of particular interest, as they were at higher risk of infections due to the administration of immunosuppressive or immune-modulatory agents. Evidence has indicated that PwMS treated with anti-CD20 monoclonal antibodies were at increased risk of severe COVID-19, making vaccination crucial for this population (5-7). However, as the COVID-19 vaccination program progressed, questions arose about the ability of PwMS on disease-modifying treatment (DMT) to mount an effective immune response after vaccination. Numerous studies have shown that anti-CD20s and sphingosine-1-phosphate receptor modulators may attenuate the humoral response to COVID-19 vaccination. However, there remains uncertainty about the effect of DMTs on cell-mediated responses and innate immunity (8-11).
Notably, Iran, as a middle-income country, has a high prevalence of MS, with an ongoing upward trend. Since 2015, a significant shift in the prescribing process of DMTs has been observed, with anti-CD20 therapies increasingly used as first-line treatments (12). In this context, individualized management has become a state-of-the-art approach provided through research to understand how to enhance the immune response to vaccination in PwMS (13).
2. Objectives
Despite extensive studies, a limited number of investigations in Iran have addressed the effect of DMTs on the effectiveness of COVID-19 vaccines. Given the importance of MS management and the accessibility and feasibility of serologic tests to assess antiviral immunity, this study aimed to investigate the effect of different DMTs on the levels of SARS-CoV-2 RBD IgG following BBIBP-CorV COVID-19 vaccination.
3. Methods
3.1. Study Design
This quasi-experimental study was conducted in 2021 at the MS clinics of Imam Hossein Hospital in Tehran and Ghaem Hospital in Mashhad. The study received Institutional Review Board approval (IR.SBMU.RETECH.REC.1400.346). Additionally, written informed consent was obtained from all participants for both participation and publication, in accordance with the Declaration of Helsinki.
3.2. Study Population
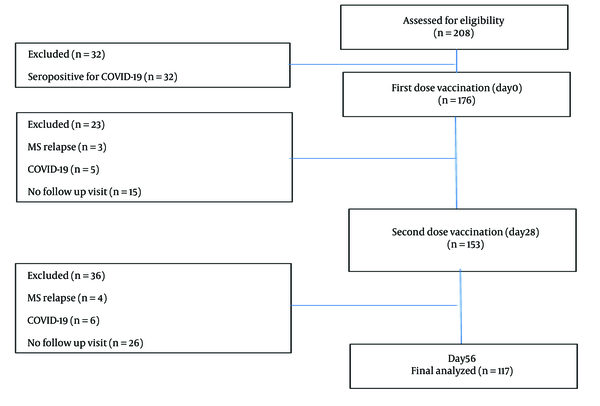
All patients meeting the following criteria were included in this study using a consecutive exposure-based sampling method: (a) a diagnosis of MS based on the McDonald Criteria 2017 and age over 18 years, with regular use of their DMT for at least six months (or nine months for glatiramer acetate); (b) no underlying disease or medication use other than for MS; (c) no active infection; and (d) a minimum interval of two months between the last infusion of rituximab and vaccination for patients treated with rituximab.
Patients were excluded if they had a history of COVID-19 infection or vaccination within six months prior to enrollment, were seropositive for COVID-19 in the first blood sampling, had a history of clinical relapse or steroid therapy in the four weeks preceding the study, were attempting to conceive, were pregnant or in the postpartum period, developed clinical relapse, COVID-19, or serious adverse events associated with the COVID-19 vaccine during the study, or did not attend regularly for vaccination and follow-up visits.
A total of 208 patients were recruited, of whom 117 eventually met the eligibility criteria (Figure 1).
3.3. Interventions and Data Collection
3.3.1. Multiple Sclerosis Characteristics
In a face-to-face interview with patients, a questionnaire was completed to collect demographic characteristics, including age, gender, Body Mass Index (BMI), MS phenotype, disease duration, DMT use, and Expanded Disability Severity Scale (EDSS) score.
3.3.2. COVID-19 Vaccination
All participants received two intramuscular injections of 4 µg of the Sinopharm BBIBP-CorV vaccine (equivalent to 0.5 mL per dose), administered 28 days apart in the deltoid muscle.
3.3.3. Blood Sampling
The first blood sample was collected just before vaccination, followed by additional samples 28 days after each vaccination.
3.3.4. Laboratory Analysis
First, we measured serum-specific IgG and IgM against the SARS-CoV-2 nucleocapsid antigen using the ELISA PISHTAZ kit (Pishtaz Teb Diagnostics, IRAN, No. MA.SARS-CoV-2 IgM_96_02). The Cut-off Index (COI) was calculated according to the mathematical method described in the kit catalog (PISHTAZ). A COI ≥ 1.1 was interpreted as reactive, while a COI < 1.1 was considered non-reactive. For patients who were non-reactive for serum-specific IgG and IgM against the SARS-CoV-2 nucleocapsid antigen, anti-RBD IgG was measured using the ELISA SARS-CoV-2 IgG DIAZIST kit (Sina Biotech, IRAN, No. DG.COVSG.01). A cut-off point ≥ 11 AU/mL was interpreted as reactive, and a cut-off point < 11 AU/mL was considered non-reactive. All samples were analyzed in a single run to minimize between-run variability.
3.4. Statistical Analysis
Continuous variables were expressed as the mean ± SD, while categorical variables were presented as percentages and COI. ANOVA was used to compare data across DMT classes. All statistical analyses were performed using GraphPad Prism Software version 8.0 (GraphPad Software, San Diego, CA, USA). A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Demographic and Clinical Data
One hundred and seventeen patients were enrolled between January 2021 and January 2022. The majority of the patients (76.1%) were female, with a mean age of 38.2 ± 9.7 years. Most patients (80.2%) were diagnosed with relapsing–remitting MS (RRMS), with a mean disease duration of 7.6 ± 8 years. The majority (92.9%) were independently ambulatory. The baseline clinical and demographic characteristics of the patients are summarized in Table 1.
| Variables | DMT | |||||||
|---|---|---|---|---|---|---|---|---|
| None (n = 6) | DMF (n = 14) | Fingolimod (n = 19) | GA (n = 7) | INF-β (n = 19) | NTZ (n = 6) | RTX (n = 38) | Teriflunomide (n = 8) | |
| Age (y) | 39.6 ± 10.1 | 32.5 ± 5.5 | 35.8 ± 8.3 | 39 ± 9.5 | 37.9 ± 10 | 31.6 ± 5.4 | 41 ± 9.3 | 51.5 ± 11.3 |
| BMI | 23.6 ± 2.6 | 24 ± 4.1 | 25.2 ± 4.1 | 26.7 ± 4.4 | 24.9 ± 3.9 | 25.6 ± 4.5 | 24 ± 3.5 | 25.1 ± 3.5 |
| MS duration (y) | 17.2 ± 10.7 | 5 ± 7.1 | 8.8 ± 7.8 | 7.7 ± 5.4 | 5.7 ± 4.4 | 4 ± 3.7 | 8.8 ± 7.4 | 12 ± 12.6 |
| Gender | ||||||||
| Female | 5 (83.3) | 10 (71.4) | 10 (52.6) | 5 (71.4) | 15 (78.9) | 6 (100) | 31 (81.6) | 7 (87.5) |
| Male | 1 (16.7) | 4 (28.6) | 9 (47.4) | 2 (28.6) | 4 (21.1) | 00 | 7 (18.4) | 1 (12.5) |
| MS phenotype | ||||||||
| RRMS | 1 (50) | 11 (91.7) | 19 (100) | 7 (100) | 16 (100) | 4 (80) | 7 (36.9) | 4 (66.7) |
| PPMS | 0 | 1 (8.3) | 0 | 0 | 0 | 0 | 2 (10.5) | 0 |
| SPMS | 1 (50) | 0 | 0 | 0 | 0 | 1 (20) | 10 (52.6) | 2 (33.3) |
| EDSS | ||||||||
| 0 - 3.5 | 00 | 11 (91.7) | 11 (73.3) | 7 (100) | 10 (66.7) | 2 (40) | 6 (31.6) | 2 (33.3) |
| 4 - 5.5 | 5 (100) | 1 (8.3) | 4 (26.7) | 0 | 5 (33.3) | 3 (60) | 9 (47.4) | 2 (33.3) |
| ≥ 6 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (21.1) | 2 (33.3) |
The Demographic and MS-related Characteristics of Patients with Multiple Sclerosis Who Received Two Dosages of the Sino Pharm Vaccine a
4.2. Vaccine Safety
The BBIBP-CorV COVID-19 vaccine was generally safe in PwMS, with no reports of serious adverse events. A total of 45 patients (38.46%) reported at least one adverse event, with myalgia being the most frequently reported. Additionally, no patient experienced a clinical relapse within six weeks after vaccination.
4.3. Humoral Immune Response
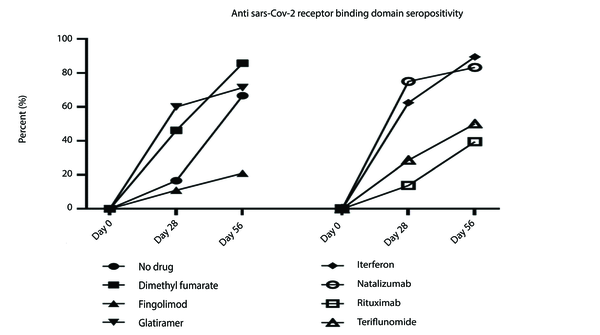
An optimal humoral immune response, defined as a 50% increase in antibody titer on the 28th day after the second vaccination, was observed as follows: INF-β (interferon beta) (89.5%), glatiramer acetate (71.4%), dimethyl fumarate (85.7%), teriflunomide (50%), fingolimod (21.1%), natalizumab (83.5%), rituximab (38.4%), and no DMT (83.3%). The results revealed a significant association between SARS-CoV-2 seroconversion and DMT class at both timelines (P = 0.046, P = 0.004). Additionally, the antibody titer was significantly related to the DMT class (P = 0.048, P = 0.000) (Figure 2).
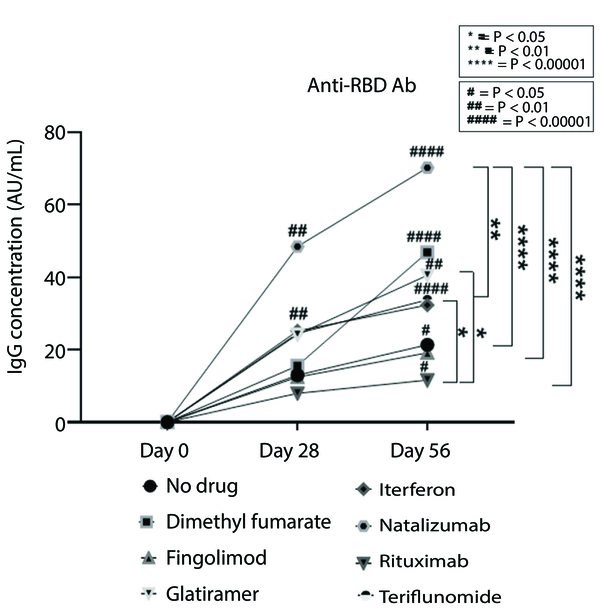
Figure 1 illustrates an increasing trend in antibody titers to SARS-CoV-2 across all DMTs, with more pronounced increases for INF-β and dimethyl fumarate during the first timeline. Furthermore, a significant difference in the final antibody titer was observed between patients treated with natalizumab and those treated with INF-β, fingolimod, and rituximab. The final antibody titer also differed significantly between patients treated with rituximab compared to those treated with INF-β and glatiramer acetate (Figure 3).
The trend of antibody levels in baseline, 28 days and 56 days after Sinopharm vaccination in Iranian PwMS treated with different DMTs. Asterisks show significant differences between each DMT group on 56 days after vaccination. Hashes are showing significant differences between day 0 and day 28 or day 56. Anti-RBD Ab, receptor binding domain antibody; PwMS, patient with multiple sclerosis; DMT, disease-modifying treatment.
Considering other possible contributing factors, we revealed no statistical association between MS phenotype (P = 0.263, P = 0.20), sex (P = 0.633, P = 0.92), age (P = 0.54, P = 0.87), and BMI (P = 0.46, P = 0.09).
5. Discussion
Vaccination against SARS-CoV-2 has significantly contributed to controlling the COVID-19 pandemic. However, there remains substantial uncertainty regarding the optimal humoral response to SARS-CoV-2 vaccination in PwMS. Previous studies have suggested that PwMS on DMTs might exhibit a reduced humoral response to the vaccine, particularly those treated with anti-CD20 therapies and fingolimod, raising critical questions about how to vaccinate immunocompromised individuals most effectively (8, 14-16).
Since the Sinopharm vaccine is the most commonly used in Iran, and limited data exist on the immune response to the COVID-19 vaccine in the Iranian population, the present study investigated the humoral response in Iranian PwMS treated with different DMTs. Patients received the Sinopharm vaccine in two stages, with evaluations conducted 28 days after the first and second doses. The results revealed that 28 days after the first dose, antibody levels in PwMS treated with teriflunomide, fingolimod, and rituximab showed no significant difference compared to baseline. However, 28 days after the second dose, an increase in humoral immunity was observed across all PwMS, regardless of the DMT type. Nevertheless, PwMS treated with fingolimod and rituximab failed to meet the minimum vaccine efficacy requirements based on WHO standards.
Our findings align with numerous reports during the COVID-19 pandemic indicating a lower humoral response in PwMS treated with fingolimod and anti-CD20 therapies (8, 14-16). The primary reason for the diminished humoral response with anti-CD20 therapies is attributed to their mechanism of action, which reduces the number of naive and memory B-cells, thereby decreasing antibody secretion (17). However, other factors, including disease duration, treatment duration, dosing interval, age, comorbidities, and BMI, may also influence the immune response (18). Several studies have demonstrated a negative association between BMI and age with vaccine immunity, while others, consistent with our results, have not found such an association (19-23). Additionally, robust evidence suggests that exposure to anti-CD20 therapies 3-6 months before COVID-19 vaccination may significantly impair the development of a protective humoral response. This is consistent with prior observations that B-cell repopulation typically begins approximately six months after the last anti-CD20 treatment (24, 25).
Despite a diminished humoral response, growing evidence suggests a preserved T-cell immune response in patients treated with anti-CD20 therapies. A recent review demonstrated that SARS-CoV-2-specific memory T-cell responses were not only comparable between BNT162b2-vaccinated healthy controls and ocrelizumab-treated PwMS, but also showed a higher level of IFN-γ-producing T-cells in the ocrelizumab group. This finding signifies an enhanced vaccine-induced T-cell response in PwMS treated with ocrelizumab (10). Similarly, another study reported preserved CD4 and CD8 T-cell responses in rituximab-treated patients with autoimmune diseases, even in those lacking a humoral response (10). However, limited data suggest reduced SARS-CoV-2-specific CD4 T-cell responses in patients treated with fingolimod, likely due to CD4 T-cell lymphopenia or disrupted T and B-cell interactions in lymph nodes (10).
The effect of teriflunomide on the immune response remains a topic of interest. While some studies have shown a mild dose-dependent reduction in the efficacy of influenza and rabies vaccines in PwMS treated with teriflunomide, others have demonstrated effective immune responses to seasonal influenza vaccination, consistent with the preservation of protective immune responses (10). Additionally, most studies on COVID-19 vaccination indicate that PwMS treated with teriflunomide are likely to mount a similar immune response to untreated patients (8, 14-16).
Considering all factors, PwMS should be encouraged to follow immunization programs, with appropriate timing for patients treated with anti-CD20 therapies and the inclusion of booster doses to achieve optimal immune responses.
Like other observational studies, our work has some limitations, the most notable being the small sample size and the lack of a control population for comparison. Additionally, we did not assess SARS-CoV-2 serostatus after the third and fourth vaccination doses. Furthermore, we evaluated only IgG responses as a measure of humoral immunity, whereas the adaptive immune response to SARS-CoV-2 depends on both cellular responses and specific antibodies. Therefore, these findings should be interpreted cautiously and generalized with care. Further studies with larger sample sizes and longer follow-up durations are needed to confirm our findings.
5.1. Conclusions
The present study revealed that PwMS treated with INF-β, glatiramer acetate, teriflunomide, dimethyl fumarate, and natalizumab produced optimal humoral immune responses after receiving two doses of the BBIBP-CorV (Sinopharm) vaccine. However, anti-CD20 therapies and fingolimod significantly reduced humoral immune responses, underscoring the need for cautious interpretation of vaccine effectiveness in these populations. This calls for a comprehensive evaluation of both B and T-cell responses, as well as consideration of booster doses in COVID-19 vaccination strategies for these patients.