1. Background
In 2019, the SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) virus was highly contagious and pathogenic, causing mild to severe respiratory failure (1). The emergence of COVID-19 for some authors represents a health public catastrophe due to the high infection rate and the risk of multiple organ involvement or death (2). The clinical spectrum of infection was wide. The most common symptoms at the COVID-19 onset were fever, cough, myalgia, fatigue, sputum production, headache, diarrhea, and dyspnea (3).
To understand the mechanism involved in COVID-19 pathogenesis, Rasool and coworkers separated routine laboratory testing into two groups. The first group evaluated the proinflammatory response consistent with cytokine storm, and the second group evaluated the progression to multi-organ damage/failure (hepatic, cardiac, renal) (4). The most investigated biomarkers could help to predict disease progression and guide treatment decisions (5-7).
In this sense, the biomarkers as lactate dehydrogenase (LDH), aspartate transaminase (AST), C-reactive protein (CRP), ferritin, D-dimer, IL-2R, IL-2, IL-10 and TNFα [2] (8, 9), white blood cell count, are measures of anticoagulation, and procalcitonin (10) to the monitoring of patients during COVID-19 infection.
The COVID-19 infection dramatically increased hospitalization costs. There are current studies suggesting that high rates of hospitalization, medications, diagnosis, and follow-up (laboratory tests and medical imaging) were the factors that increased the costs of management during the pandemic (11, 12). However, it has been reported that new prognostic parameters can help quickly and cheaply identify patients at risk for severe SARS-CoV-2 infection quickly and cheaply (13). Concerning these issues, the routine blood count is cheap and extremely important in predicting the prognosis of severe COVID-19 patients (14). Although some researchers and expert clinicians suggested that systemic immune-inflammatory biomarkers highlighted the potential role of the neutrophil-lymphocyte ratio (NLR), and the platelet-lymphocyte ratio (PLR) represented a rapid, easy, widely available, and relatively inexpensive tool (15, 16) that can serve as a valuable predictor of hospital mortality and has been proposed as a new biomarker for systemic inflammation (17, 18).
Unfortunately, many patients were hospitalized with COVID-19 pneumonia in Mexico, and we faced higher costs for management.
2. Objectives
Hence, the primary objective of our study was to calculate the NLR and the PLR and determine the relation to the prognosis of COVID-19 patients in the Mexican Southeast.
3. Methods
Following ethics criteria, this study was recorded in the Australian New Zealand Clinical Trial Register (ACTRN12620001265965) and the ethics committee of Dr. Juan Graham Casasús Hospital (CEIJGC-2023-04). This retrospective study included 189 records from patients admitted to Dr. Juan Graham Casasús Hospital at Villahermosa, Tabasco, México, SARS-CoV-2 infection confirmed by PCR between June 2020 and January 2022. As it was of a retrospective nature, the informed consent was waived.
3.1. Data Collection
We extracted epidemiological, clinical, and laboratory data from electronic medical records. The epidemiological variables included age and sex. The clinical variables included comorbid conditions, symptoms at admission, number of hospital days, and discharge status. Finally, we examined the clinical laboratory variables: Complete blood count, blood chemistry analyses, coagulation test, levels of ferritin, Dimer-D, CRP, LDH, and interleukin-6 (IL-6).
We calculated the Systemic Immune-Inflammation Index following formulas: Neutrophil Lymphocyte Ratio: Absolute neutrophil count/the absolute lymphocyte count, PLR: Absolute platelet count/absolute lymphocyte count, Systemic inflammatory Index (SII): (Absolute platelet count) (absolute neutrophil count)/absolute lymphocyte count.
3.2. Statistical Analysis
Statistical analyses were performed using the SPSS software (version 25). Participants’ epidemiological, clinical, and laboratory data were summarized with descriptive statistics. The continuous variables were expressed as average and compared with the Student t-test, and categorical variables were expressed as numbers and percentages. These were compared with the χ2 test, which was a test between the survivor group and the non-survivor group. P-values less than 0.05 were considered significant.
The sensitivity and specificity in the prediction of death, according to the cutoff values of the NLR, PLR, and SII, were determined by the ROC curve. Then, the patient’s data were divided into two groups according to the cutoff value (lower than the cutoff and higher than the cutoff).
4. Results
We admitted 189 patients with confirmed SARS-CoV-2 who were hospitalized at the Dr. Juan Graham Casasús Hospital. The average age was 61.82 ± 17.3 years. Most of the included patients were men, 59.8% (n = 113). The main symptom was cough; hypertension was the most common comorbidity. The frequency of death was 55.6% (n = 105). The rest of the features are listed in Table 1.
| Features | Values |
|---|---|
| Age | 61.82 ± 17.3 |
| Male (n = 113) | 59.8 |
| Symptoms | |
| Fever (n = 123) | 65.1 |
| Cough (n = 147) | 77.8 |
| Sore throat (n = 48) | 25.4 |
| Dyspnea (n = 128) | 67.7 |
| Chest pain (n = 54) | 28.6 |
| Headache (n = 129) | 68.3 |
| Myalgias (n = 79) | 41.8 |
| Arthralgias (n = 76) | 40.2 |
| Runny nose (n = 38) | 20.1 |
| Comorbidities | |
| Diabetes (n = 61) | 32.3 |
| Hypertension (n = 76) | 40.2 |
| CKD (n = 14) | 7.4 |
| Obesity (n = 42) | 22.2 |
| Death (n = 105) | 55.6 |
| Hospital stay (d) | 11.7 ± 11.2 |
Abbreviation: CKD, chronic kidney disease.
a Values are expressed as % or mean ± SD.
The laboratory results were obtained at hospital admission, and comparisons between survivors and non-survivors are presented in Table 2. The comparison of survivors and non-survivors showed a significant difference among age (65.6 vs. 56.9 P = 0.001), white blood cell count (14.1 vs. 10.2 P = 0.001), neutrophil (12.4 vs. 8.5 P = 0.002), CRP (213.7 vs. 158.5 P = 0.003), IL-6 (192.3 vs. 81.0 P = 0.005), NLR (23.5 vs. 11.4 P = 0.000), PLR (524.8 vs. 287.6 P = 0.010), SII (6982.0 vs 3003.1 P = 0.004).
| Variables | Values | Discharge | P-Value | |
|---|---|---|---|---|
| Non-survivors | Survivors | |||
| Age (y) | 61.82 ± 17.3 | 65.6 ± 16.2 | 56.9 ± 16.9 | 0.001 b |
| Gender (%) | 0.457 | |||
| Male (n = 113) | 59.8 | 64 | 49 | |
| Female (n = 76) | 40.2 | 41 | 34 | |
| Red blood cell count (106/µL) | 4.2 ± 0.9 | 4.1 ± 1.0 | 4.3 ± 0.8 | 0.115 |
| Hemoglobin (g/dL) | 12.4 ± 2.7 | 12.2 ± 2.9 | 12.8 ± 2.3 | 0.138 |
| Hematocrit (%) | 37.7 ± 8.0 | 37.1 ± 8.7 | 38.6 ± 6.7 | 0.223 |
| White blood cell count (103/µL) | 12.3 ± 8.3 | 14.1 ± 9.6 | 10.2 ± 5.4 | 0.001 b |
| Lymphocyte count (103/µL) | 1.7 ± 6.7 | 2.0 ± 8.6 | 1.3 ± 2.9 | 0.501 |
| Neutrophil (103/µL) | 10.6 ± 8.6 | 12.4 ± 10.2 | 8.5 ± 5.4 | 0.002 b |
| Platelet count (103/µL) | 245.9 ± 172.6 | 255.8 ± 204.8 | 234.8 ± 121.4 | 0.409 |
| CRP (mg/L) | 189.4 ± 119.4 | 213.7 ± 122.2 | 158.5 ± 109.6 | 0.003 b |
| Glucose (mg/dL) | 177.7 ± 101.3 | 180.3 106.1± | 175.4 ± 95.5 | 0.743 |
| Urea (mg/dL) | 61.1 ± 52.1 | 67.5 ± 53.9 | 53.4 ± 49.1 | 0.067 |
| BUN | 28.5 ± 24.4 | 31.4 ± 25.8 | 24.9 ± 22.9 | 0.072 |
| Creatinine (mg/dL) | 2.0 ± 5.5 | 2.4 ± 7.1 | 1.5 ± 2.6 | 0.293 |
| ALT (Ul/L) | 50.8 ± 69.4 | 47.8 ± 78.3 | 55.2 ± 56.4 | 0.483 |
| AST (Ul/L) | 73.8 ± 150.1 | 82.6 ± 195.7 | 62.5 ± 48.6 | 0.378 |
| Alkaline phosphatase (Ul/L) | 125.9± 91.6 | 126.6 ± 86.8 | 122.4 ± 95.7 | 0.764 |
| LDH (Ul/L) | 480.7 ± 356.1 | 578.6 ± 434.8 | 548.8 ± 181.9 | 0.000 b |
| Fibrinogen | 563.3 ± 211.9 | 580.6 ± 227.7 | 548.8 ± 181.9 | 0.384 |
| D-dimer (mg/L) | 4.6 ± 13.3 | 6.6 ± 15.5 | 2.1 ± 9.4 | 0.033 b |
| Prothrombin time (s) | 13.2 ± 3.9 | 13.8 ± 5.0 | 12.4 ± 1.1 | 0.022 b |
| aPTT (s) | 34.3 ± 15.7 | 34.1 ± 12.5 | 34.1 ± 18.9 | 0.993 |
| Serum ferritin (ng/mL) | 743.0 ± 933.4 | 851.9 ± 1099.3 | 603.2 ± 654.3 | 0.103 |
| Procalcitonin (ug/L) | 1.4 ± 5.1 | 2.0 ± 6.4 | 0.6 ± 2.3 | 0.090 |
| IL-6 (pg/mL) | 145.2 ± 228.7 | 192.3 ± 258.7 | 81.0 ± 160.5 | 0.005 b |
| NLR | 18.1 ± 20.0 | 23.5 ± 24.1 | 11.4 ± 9.7 | 0.000 b |
| PLR | 418.3 ± 626.4 | 524.8 ± 809.2 | 287.6 ± 190.1 | 0.010 b |
| SII | 5198.7 ± 9340.9 | 6982.0 ± 11877.8 | 3003.1 ± 377.8 | 0.004 b |
Abbreviations: CRP, C Reactive Protein; BUN, Blood Urea Nitrogen; ALT, Alanine transaminase; AST, Aspartate transaminase; aPTT, activated partial thromboplastin time; LDH, Lactate dehydrogenase; NLR, Neutrophil-Lymphocyte Ratio; PLR, Platelet Lymphocyte Ratio; SII, Systemic Inflammatory Index.
a Values are expressed as mean ± SD unless otherwise indicated.
b P < 0.05.
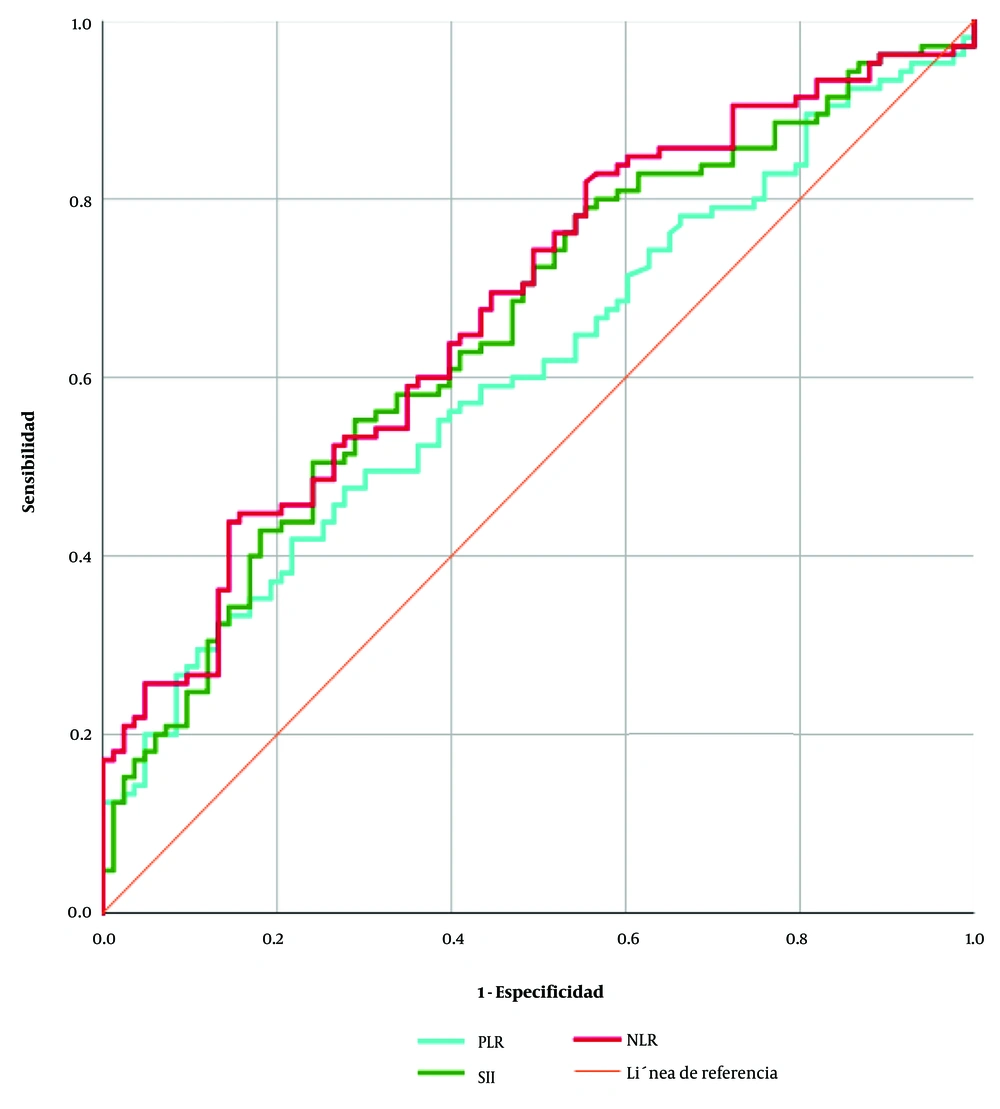
Figure 1 shows that the optimal cutoff for the NLR was of 8.6 with a sensitivity of 70.4% and a specificity of 51.8% (AUC 0.678, CI 95% 0.6024 - 0.7541, P < 0.0001), the optimal cutoff for the PLR was of 252.6 with a sensitivity of 60.0% and a specificity of 50.6% (AUC 0.6096, CI 95% 0.5297 - 0.6895, P < 0.0099), and the optimal cutoff for the SII was of 1815 with a sensitivity of 70.4% and a specificity of 51.8% (AUC 0.6566, CI 95% 0.5788 - 0.7344, P < 0.0002).
Among all the cases studied, the 60.3% (n = 114) had NLR > 8.6. Table 3 shows the comparison of the NLR < 8.6 and > 8.6. There was a significant difference in age (58.2 ± 17.1 vs. 64.1 ± 16.6 P = 0.021), hospital stay (99.6 ± 9.6 vs. 13.1 ± 11.9 P = 0.033), gender (male 37 vs. 76 P = 0.017), white blood cell count (9.0 ± 10.4 vs. 14.5 ± 5.5 P < 0.000), CRP (136.7 ± 84.9 vs. 224.7 ± 126.3 P < 0.000), urea (48.1 ± 33.3 vs. 69.6 ± 60.0 P = 0.005), BUN (22.1 ± 15.4 vs. 32.6 ± 28.0 P = 0.003), LDH (411.2 ± 237.9 vs., 529.5 ± 413.9 P = 0.0029), fibrinogen (456.7 ± 161.4 vs. 634.3 ± 212.6 P = 0.000), IL-6 (91.3 ± 166.9 vs. 180.7 ± 256.4 P = 0.025) and death (31 vs. 74 P = 0.002).
| Variables | NLR | P-Value | |
|---|---|---|---|
| < 8.6 | > 8.6 | ||
| Age (y) | 58.2 ± 17.1 | 64.1 ± 16.6 | 0.021 b |
| Hospital stay (d) | 9.6 ± 9.6 | 13.1 ± 11.9 | 0.033 b |
| Male | 37 (49.3) | 76 (66.7) | 0.017 b |
| Red blood cell count (106/µL) | 4.2 ± 0.9 | 4.2 ± 0.8 | 0.715 |
| Hemoglobin (g/dL) | 12.5 ± 2.7 | 12.4 ± 2.6 | 0.725 |
| Hematocrit (%) | 38.0 ± 8.0 | 37.5 ± 8.0 | 0.686 |
| White blood cell count (103/µL) | 9.0 ± 10.4 | 14.5 ± 5.5 | 0.000 b |
| Lymphocyte count (103/µL) | 3.3 ± 10.5 | 0.6 ± 0.3 | 0.006 b |
| Neutrophil (103/µL) | 5.6 ± 3.7 | 14.0 ± 9.3 | 0.000 b |
| Platelet count (103/µL) | 193.5 ± 106.4 | 280.3 ± 197.9 | 0.001 b |
| CRP (mg/L) | 136.7 ± 84.9 | 224.7 ± 126.3 | 0.000 b |
| Glucose (mg/dL) | 172.5 ± 106.6 | 181.1 ± 98.1 | 0.571 |
| Urea (mg/dL) | 48.1 ± 33.3 | 69.6 ± 60.0 | 0.005 b |
| BUN | 22.1 ± 15.4 | 32.6 ± 28.0 | 0.003 b |
| Creatinine (mg/dL) | 1.4 ± 1.9 | 2.4 ± 6.9 | 0.215 |
| ALT (Ul/L) | 47.7 ± 43.7 | 52.8 ± 82.9 | 0.647 |
| AST (Ul/L) | 75.9 ± 111.9 | 72.3 ± 172.3 | 0.877 |
| Alkaline phosphatase (Ul/L) | 125.7 ± 87.9 | 126.1 ± 94.6 | 0.974 |
| LDH (UI/L) | 411.2 ± 237.9 | 529.5 ± 413.9 | 0.029 b |
| Fibrinogen | 456.7 ± 161.4 | 634.3 ± 212.6 | 0.000 b |
| D-Dimer (mg/L) | 3.7 ± 11.7 | 5.2 ± 14.2 | 0.465 |
| Prothrombin time (s) | 12.9 ± 2.3 | 13.5 ± 4.7 | 0.340 |
| aPTT (s) | 34.4 ± 11.1 | 34.2 ± 18.3 | 0.930 |
| Serum ferritin (ng/mL) | 626.7 ± 629.2 | 817.2 ± 1080.7 | 0.218 |
| Procalcitonin (ug/L) | 0.69 ± 1.8 | 1.8 ± 6.4 | 0.0149 |
| IL-6 (pg/mL) | 91.3 ± 166.9 | 180.7 ± 256.4 | 0.025 b |
| PLR | 162.6 ± 90.1 | 586.6 ± 758.6 | 0.000 b |
| SII | 921.7 ± 718.5 | 921.7 ± 11166.7 | 0.000 b |
| Death (N = 105) | 31 (41.9) | 74 (64.9) | 0.002 b |
Abbreviations: CRP, C reactive protein; BUN, blood urea nitrogen; ALT, alanine transaminase; AST, aspartate transaminase; aPTT, activated partial thromboplastin time; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio, PLR, platelet lymphocyte ratio; SII, systemic Inflammatory Index.
a Values are expressed as mean ± SD or No. (%).
b P < 0.05.
A total of 55.0% (n = 104) had PLR > 252.6. Appendices 1 and 2 in Supplementary File show the comparison of PLR < 252.6 and > 252.6. There was a significant difference among hospital stay (9.5 ± 9.8 vs. 13.5 ± 11.9 P = 0.012), gender (male 44 vs. 69 P = 0.042), white blood cell count (10.9 ± 10.3 vs. 13.5 ± 5.9 p = 0.030), lymphocyte count (3.1 ± 9.8 vs. 0.6 ± 0.3 P = 0.010), neutrophil (8.6 ± 10.8 vs. 12.3 ± 5.8 P = 0.003), platelet count (176.7 ± 85.5 vs. 302.4 ± 203.1 P = 0.000), CRP (163.0 ± 114.9 vs. 211.1 ± 119.3 P = 0.010), fibrinogen (458.9 ± 178.3 vs. 651.2 ± 198.5 P = 0.000), NLR (7.3 ± 6.6 vs 27.0 ± 22.7.4 P = 0.000) and SII (1271.0 ± 1186.3 vs 8408.9 ± 11617.8 P = 0.000).
We also examined the comparison of SII < 1815, and >1815, 60.3% (n = 114) had SII >1815, there was a significant difference among age (57.3 ± 17.2 vs. 64.7 ± 16.3 P = 0.004), hospital stay (9.3 ± 10.1 vs. 13.3 ± 11.6 P = 0.018), gender (male 37 vs. 76 P = 0.017), white blood cell count (8.7 ± 10.4 vs. 14.7 ± 5.3 P = 0.000), lymphocyte count (3.3 ± 10.5 vs. 0.7 ± 0.4 P = 0.009), neutrophil (5.4 ± 3.5 vs. 14.1 ± 9.2 P = 0.000), platelet count (168.3 ± 83.3 vs. 297.0 ± 196.0 P = 0.000), CRP (145.2 ± 101.0 vs. 218.3 ± 122.1 P = 0.000), urea (50.5 ± 40.9 vs. 68.0 ± 57.4 P = 0.024), BUN (23.2 ± 19.0 vs. 31.9 ± 26.8 P = 0.017), fibrinogen (451.4 ± 176.9 vs. 635.7 ± 201.8 P = 0.000), IL6 (87.3 ± 170.9 vs. 183.3 ± 253.7 P = 0.016) NLR (5.3 ± 4.7 vs. 26.5 ± 21.7 P = 0.000) and PLR (155.5 ± 87.1 vs. 591.3 ± 756.2 P = 0.000) and death (31 vs. 74 P = 0.002) (Appendix 2 in Supplementary File).
5. Discussion
We aimed to understand the role of NLR in the prognosis of COVID-19 patients in the Mexican Southeast. Here, it was highlighted that age, white blood cell count, neutrophil, CRP, LDH, d-dimer, prothrombin time, IL-6, NLR, PLR, and SII differed between survivors and non-survivors.
In general, the results show that the age, white blood cell count, neutrophil, CRP, IL-6, NLR, PLR, and SII were significantly higher in the non-survivor group. In agreement with the previous reports of age, Il-6, and the CRP, there was a strong association with respiratory failure (19). Likewise, leukocytosis and lymphopenia, high levels of ALT, LDH, D-dimer and ferritin, IL-2R, IL-8, IL-10, and TNF-α were more common in severe cases (2, 6, 20).
In hospitalized patients with COVID-19 in Southeast Mexico, we have found that higher NLR shows significant differences within older age, hospital stay, gender, white blood cell count, CRP, urea, BUN, LDH, fibrinogen, IL-6, and death. We emphasized similarities with a report performed on the Chinese population, where 245 admitted patients, and found that the NLR was higher. This was significantly associated with an increased risk of death during hospitalization (17).
There are currently some published studies with different levels of evidence about the role of NLR in the prognosis of patients with COVID-19 (14, 20, 21). As noted in a recent review, NLR may improve its usefulness to clinicians in assessing the patient´s condition and the severity of inflammation in the course of COVID-19. It was suggested that higher NLR is related to patients who died (22). Now, it is recognized that the elevated NLR was an independent prognostic biomarker that concerned pneumonia progression in COVID-19 patients (23, 24).
Studies have reported that NLR showed high sensitivity and specificity for prognosis of COVID-19 (25, 26). In this study, we also found that NLR showed a sensitivity of 70.4% and a specificity of 51.8% (AUC 0.678, IC 0.6024 - 0.7541, P < 0.0001) at a cutoff of 8.6. This demonstrates a potential utility in detecting differences among cases in our population. Another study had already demonstrated that the ideal cutoff values for NLR were 8.4 with 83.6% specificity and 80.4% sensitivity (26). So far, there are different reports concerning the cutoff of NLR. In patients from Cairo, it was reported that there was a 52.7% sensitivity and an 82.9% specificity at a cutoff of 2.5 (25). In other research, the threshold was at 3.3 for NLR; It showed a superior prognostic possibility of clinical symptoms, which were changing from mild to severe (24). More specifically, the optimal cutoff for NLR was 5.2, which was an independent predictor of the requirement for admission to a critical care unit (20).
The findings from this analysis also suggest that PLR and SII would help to identify prognosis and risk of death (22, 27, 28). In this study, we also found that the PLR > 252.6 and SII > 1815 showed significant differences in markers related to diagnosis, severity, or mortality in COVID-19 patients.
In other pathologies, a higher NLR value at hospital admission was associated with mortality in solid cancer patients (29-31) with Hodgkin lymphoma (32), in acute ischemic stroke patients who were receiving reperfusion therapy. The NLR predicted clinical outcomes (33). Also, prior studies have defined elevated NLR as associated with an increased risk of developing persistent organ failure in patients with hypertriglyceridemic pancreatitis (34). Regarding the available literature, NLR and SII exhibit capabilities for postoperative pneumonia in elderly hip fracture patients (35). Likewise, the inflammatory biomarkers NLR, PLR, and SII have been suggested as a potential prognostic tool for depression and its severity (36), diabetic microvascular complications (37), and predicting severe forms of acute cholecystitis (38).
Our study has some notable limitations. Firstly, this study was retrospective in nature and cross-sectional design. Secondly, the data extraction was in a single center, the Dr. Juan Graham Casasús Hospital. Thirdly, our study included 189 patients for evaluation of the new systemic inflammation markers from results found at hospital admission but did not include the dynamic during the hospital stay.
In conclusion, we propose that NLR, PLR, and SII should be considered an inexpensive, accessible, and effective biomarker in the management of patients with COVID-19. The age, hospital stay, gender, white blood cell count, CRP, urea, BUN, LDH, fibrinogen, IL-6, and death had significant differences with elevated inflammatory indexes. Finally, the high NLR, PLR, and SII were related to poor prognosis in COVID-19 patients.