1. Background
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19), which emerged in Wuhan, China, in December 2019. Within two months, it spread globally and escalated into a pandemic (1, 2). COVID-19 is highly transmissible through inhalation of infected aerosols. After an incubation period of 3 to 14 days, it may result in a wide range of clinical manifestations, from asymptomatic cases to severe, potentially fatal illness. COVID-19 presents a complex pathology, affecting various organs and involving oxidative stress and inflammatory responses (3). It is incorrect to assume that all COVID-19 patients recover within two weeks; many non-hospitalized patients with mild severity experience persistent symptoms. However, the long-term consequences of COVID-19 remain poorly understood (4).
There is no consensus on a precise definition of post-acute COVID-19 syndrome, which generally refers to individuals who have recovered from COVID-19 but continue to experience prolonged or persistent symptoms (5). When COVID-19 symptoms last longer than three weeks from onset, or persist up to twelve weeks, this is termed post-acute COVID-19 syndrome (6). These symptoms can include fatigue, dyspnea, cough, arthralgia, chest pain, headache, myalgia, palpitations, anosmia, ageusia, memory and concentration issues, skin rashes, and hair loss (7). Researchers suggest that a reduced antibody response to SARS-CoV-2 infection, along with a prolonged inflammatory response, may contribute to post-acute COVID-19 syndrome (8). Fatigue remains a prominent symptom among COVID-19 patients even twelve weeks post-recovery, with many individuals unable to resume their previous activity levels (9). Dyspnea is another common symptom, for which potential causes, such as pulmonary embolism, lung fibrosis, or cardiac failure, are considered, though in most cases, no definitive cause is identified (10).
Managing post-acute COVID-19 syndrome remains a clinical challenge due to the absence of evidence-based guidelines. Vitamin B1 plays a crucial role in the tricarboxylic acid cycle. Deficient tricarboxylic acid cycle functioning, due to a lack of vitamin B1, leads to significant depletion of naive B cells. Adequate dietary intake of vitamin B1 is essential to maintain naive B cell populations for an effective immune response to antigenic stimuli (11). A well-functioning immune system is currently the most critical defense against COVID-19, with vitamins playing a supportive role in immune system efficiency. Insufficient vitamin levels can impair immune function, increasing susceptibility to infection. Vitamins strengthen physical barriers, cellular immunity, and antibody production, enhancing the body's immune defense (11).
2. Objectives
Given the limited evidence, this study was undertaken to investigate the efficacy of vitamin B1 in alleviating post-acute COVID-19 symptoms.
3. Methods
3.1. Study Design and Participants
This open-label randomized controlled trial was conducted on patients with post-acute COVID-19 syndrome who presented at Labbafi Nejad Hospital in Tehran, Iran, from October 2021 to November 2023. The study protocol was approved by the Iranian Registry of Clinical Trials (IRCT20221229056979N1). Inclusion criteria included: Patients diagnosed with post-acute COVID-19 syndrome, individuals who had recovered from COVID-19 but continued to experience persistent symptoms such as fatigue, sleep disturbances, anosmia, ageusia, chest pain, cough, arthralgia, hair loss, skin rashes, and other symptoms three weeks after symptom onset; age over 18 years; and willingness to participate in the study. Exclusion criteria included: A history of chronic disorders (e.g., endocrinopathies, dermatological diseases, neurological disorders), pregnancy or breastfeeding, hypersensitivity to thiamine (vitamin B1), and inability to complete the treatment course or follow-up.
3.2. Data Collection
In this study, data were collected using the census method. Initially, the study objectives were explained to eligible patients, and informed consent forms were completed. Baseline characteristics, including age, gender, Body Mass Index (BMI), the interval from symptom onset, systolic and diastolic blood pressure, pulse rate, respiratory rate, body temperature, oxygen saturation, and corticosteroid administration, were recorded upon admission. Additionally, laboratory findings, including white blood cell count (WBC), hemoglobin (Hb), platelet count (PLT), neutrophil percentage, lymphocyte percentage, and C-reactive protein (CRP), along with lung computed tomography (CT) scan reports from the first day, were reviewed and documented on checklists by a research team member.
3.3. Study Procedures
Patients were randomized using a simple random method with a random number table and assigned to two groups—intervention and control—with an allocation ratio of 1: 1. The intervention group received oral tablets of vitamin B1 (600 mg daily for 8 weeks, produced by Exir Pharmaceutical Company, Iran) along with supportive therapy (e.g., vitamin C, famotidine, and zinc). The control group received only the supportive therapy prescribed for the intervention group. This study was conducted as an open-label trial without blinding.
3.4. Study Outcomes
A trained physician conducted weekly interviews with the patients over nine weeks to assess symptoms of post-acute COVID-19 syndrome (e.g., ageusia, anosmia, anorexia, fatigue, sleep disorders, hair loss, concentration disorders). Patients rated the severity of these symptoms using a Visual Assessment Tool (VAT) on a scale from 0 to 10. Sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI), a questionnaire with 19 items that assess sleep quality over the past month. A total PSQI score of 6 or higher indicates poor sleep quality (Supplementary File 1). The validity and reliability of the Persian version of the PSQI have been confirmed by Farrahi Moghaddam et al., with a Cronbach’s alpha of 0.77 (12).
3.5. Statistical Analysis
Data analysis was conducted using SPSS software version 22. Qualitative variables were presented as frequency (%), while quantitative variables were described as mean ± standard deviation (SD). Comparisons between groups were made using the Independent-sample t-test for quantitative variables and the chi-square test for qualitative variables. The significance level for this study was set at P < 0.05.
3.6. Ethical Considerations
The study protocol was approved by the Ethics Committee of the School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.MSP.REC.1400.286). All procedures were conducted in accordance with the Helsinki Declaration of 2000.
4. Results
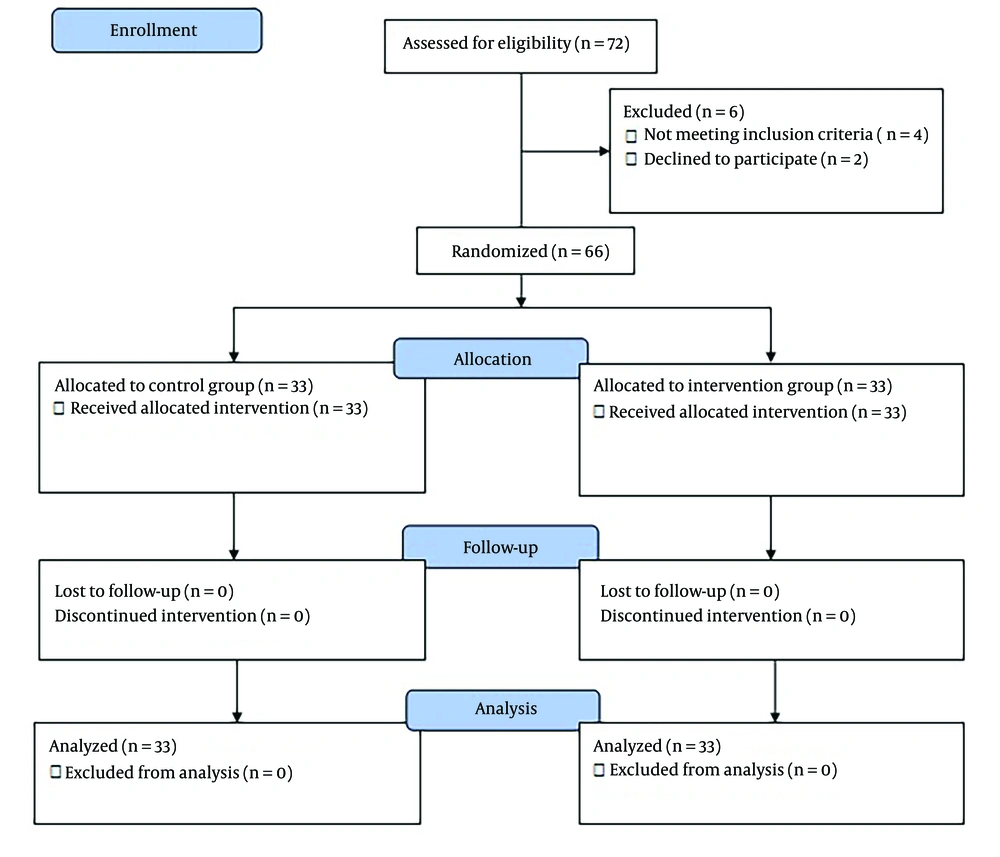
Figure 1 shows the CONSORT flow diagram of the study. After assessing eligibility among 72 patients, 66 individuals were randomized and equally assigned to the intervention and control groups (33 participants in each group). All participants continued with follow-up through the final analysis.
Table 1 presents the baseline characteristics of the patients. According to the findings, there were no significant differences between the groups regarding age (P = 0.579), gender (P = 0.805), BMI (P = 0.466), systolic blood pressure (P = 0.325), diastolic blood pressure (P = 0.084), pulse rate (P = 0.486), respiratory rate (P = 0.553), body temperature (P = 0.212), oxygen saturation (P = 0.212), WBC count (P = 0.586), hemoglobin level (P = 0.882), platelet count (P = 0.173), lung CT-scan findings (P = 0.176), and corticosteroid use (P = 0.151). However, the interval from symptom onset (P < 0.0001), neutrophil percentage (P < 0.0001), lymphocyte percentage (P = 0.007), and CRP levels (P = 0.003) differed significantly between the groups.
| Variables and Sub-group | Intervention Group; (n = 33) | Control Group; (n = 33) | P-Value |
|---|---|---|---|
| Age, (y) | 50.30 ± 12.93 | 48.39 ± 14.82 | 0.579 |
| Gender | 0.805 | ||
| Male | 18 (54.5) | 17 (51.5) | |
| Female | 15 (45.5) | 16 (48.5) | |
| BMI (kg/m2) | 26.24 ± 6.93 | 25.24 ± 3.62 | 0.466 |
| Interval from symptom onset (w) | 6.30 ± 0.95 | 8.24 ± 0.90 | > 0.0001 |
| Systolic blood pressure (mmHg) | 113.79 ± 14.53 | 110.15 ± 15.23 | 0.325 |
| Diastolic blood pressure (mmHg) | 73.79 ± 8.66 | 67.36 ± 19.19 | 0.084 |
| Pulse rate (/min) | 94.82 ± 7.11 | 93.52 ± 7.96 | 0.486 |
| Respiratory rate (/min) | 21.21 ± 3.80 | 20.61 ± 4.43 | 0.553 |
| Body temperature (axillary) | 37.72 ± 0.82 | 37.40 ± 1.22 | 0.212 |
| Oxygen saturation (%) | 95.76 ± 1.69 | 96.21 ± 1.19 | 0.212 |
| WBC (103 cells/uL) | 4969.70 ± 1818.05 | 4722.42 ± 1849.86 | 0.586 |
| Hb (mg/dL) | 14.88 ± 1.62 | 14.82 ± 1.84 | 0.882 |
| PLT (103 cells/uL) | 259507.88 ± 129471.38 | 298563.64 ± 98854.04 | 0.173 |
| Neutrophil (%) | 84.06 ± 2.62 | 80.79 ± 2.11 | > 0.0001 |
| Lymphocyte (%) | 14.88 ± 2.64 | 16.52 ± 2.09 | 0.007 |
| CRP (mg/dL) | 12.84 ± 25.38 | 16.25 ± 10.94 | 0.003 |
| Lung CT scan manifestations on the first day | 0.176 | ||
| Failed | 2 (6.1) | 9 (27.3) | |
| Mild involvement | 14 (42.4) | 6 (18.2) | |
| Moderate involvement | 5 (15.2) | 10 (30.3) | |
| Severe involvement | 1 (3.0) | 1 (3.0) | |
| Normal | 11 (33.3) | 7 (21.2) | |
| Corticosteroid administration | 0.151 | ||
| Yes | 3 (10.3) | 9 (27.3) | |
| No | 25 (86.2) | 24 (72.7) |
Abbreviations: WBC, white blood cell count; Hb, hemoglobin; PLT, platelet count.
a Values are expressed as mean ± SD or No. (%).
Table 2 presents the severity of post-acute COVID-19 syndrome from the first to the third week. In these initial three weeks, all symptoms, except for cough (P = 0.038), headache (P = 0.022), fatigue (P = 0.002), and sleep disturbances (P = 0.003), did not show significant differences between the groups. However, patients receiving vitamin B1 reported higher scores for cough, headache, fatigue, and sleep disturbances during this period. By the sixth week, the intervention group showed complete improvement in symptoms such as dyspnea (P < 0.0001), chest pain (P < 0.0001), palpitations (P < 0.0001), skin rashes (P < 0.0001), and hair loss (P < 0.0001), while these symptoms persisted in the control group. Additionally, symptoms including myalgia (P = 0.009), anosmia (P = 0.009), anorexia (P = 0.001), fatigue (P = 0.001), and sleep disturbances (P = 0.022) significantly improved in the intervention group. Table 3 provides an overview of the severity of post-acute COVID-19 syndrome in the sixth week.
| Symptoms | Intervention Group; (n = 33) | Control Group; (n = 33) | P-Value |
|---|---|---|---|
| Myalgia | 6.00 ± 1.52 | 5.36 ± 0.91 | 0.72 |
| Cough | 5.48 ± 1.57 | 4.59 ± 1.55 | 0.038 |
| Dyspnea | 4.00 ± 1.31 | 3.65 ± 0.86 | 0.370 |
| Headache | 4.91 ± 2.07 | 3.57 ± 1.08 | 0.022 |
| Ageusia | 4.96 ± 1.15 | 4.80 ± 0.79 | 0.698 |
| Anosmia | 4.90 ± 0.57 | 4.90 ± 0.57 | > 0.999 |
| Anorexia | 5.73 ± 1.46 | 5.59 ± 1.04 | 0.674 |
| Fatigue | 6.00 ± 1.26 | 4.96 ± 0.99 | 0.002 |
| Arthralgia | 4.43 ± 1.40 | 3.40 ± 0.55 | 0.153 |
| Chest pain | 3.00 ± 0.00 | 3.00 ± 2.00 | > 0.999 |
| Palpitations | 4.33 ± 0.58 | 4.00 ± 0.84 | 0.623 |
| Sleep disorders | 5.50 ± 0.52 | 4.80 ± 0.42 | 0.003 |
| Concentration disorder | 4.89 ± 0.60 | 4.75 ± 0.87 | 0.685 |
| Skin rash | 3.20 ± 0.84 | 2.80 ± 0.84 | 0.471 |
| Hair loss | 4.75 ± 0.50 | 4.50 ± 0.55 | 0.486 |
a Values are expressed as mean ± SD.
| Symptoms | Intervention Group; (n = 33) | Control Group; (n = 33) | P-Value |
|---|---|---|---|
| Myalgia | 1.12 ± 0.35 | 1.75 ± 0.46 | 0.009 |
| Cough | 1.00 ± 0.00 | 1.50 ± 0.71 | 0.423 |
| Dyspnea | 0.00 ± 0.00 | 0.50 ± 0.21 | < 0.0001 |
| Headache | 1.00 ± 0.00 | 0.73 ± 1.11 | < 0.0001 |
| Ageusia | 2.40 ± 1.14 | 2.00 ± 0.63 | 0.479 |
| Anosmia | 2.29 ± 1.11 | 3.60 ± 0.70 | 0.009 |
| Anorexia | 1.29 ± 0.49 | 2.63 ± 0.91 | 0.001 |
| Fatigue | 1.33 ± 0.52 | 2.31 ± 0.62 | 0.001 |
| Arthralgia | 1.00 ± 0.00 | 0.20 ± 0.05 | < 0.0001 |
| Chest pain | 0.00 ± 0.00 | 0.08 ± 0.02 | < 0.0001 |
| Palpitations | 0.00 ± 0.00 | 1.50 ± 0.55 | < 0.0001 |
| Sleep disorders | 1.57 ± 0.98 | 2.50 ± 0.53 | 0.022 |
| Concentration disorder | 1.50 ± 1.00 | 2.25 ± 0.62 | 0.093 |
| Skin rash | 0.00 ± 0.00 | 1.30 ± 0.72 | < 0.0001 |
| Hair loss | 0.00 ± 0.00 | 2.00 ± 0.00 | <0.0001 |
a Values are expressed as mean ± SD.
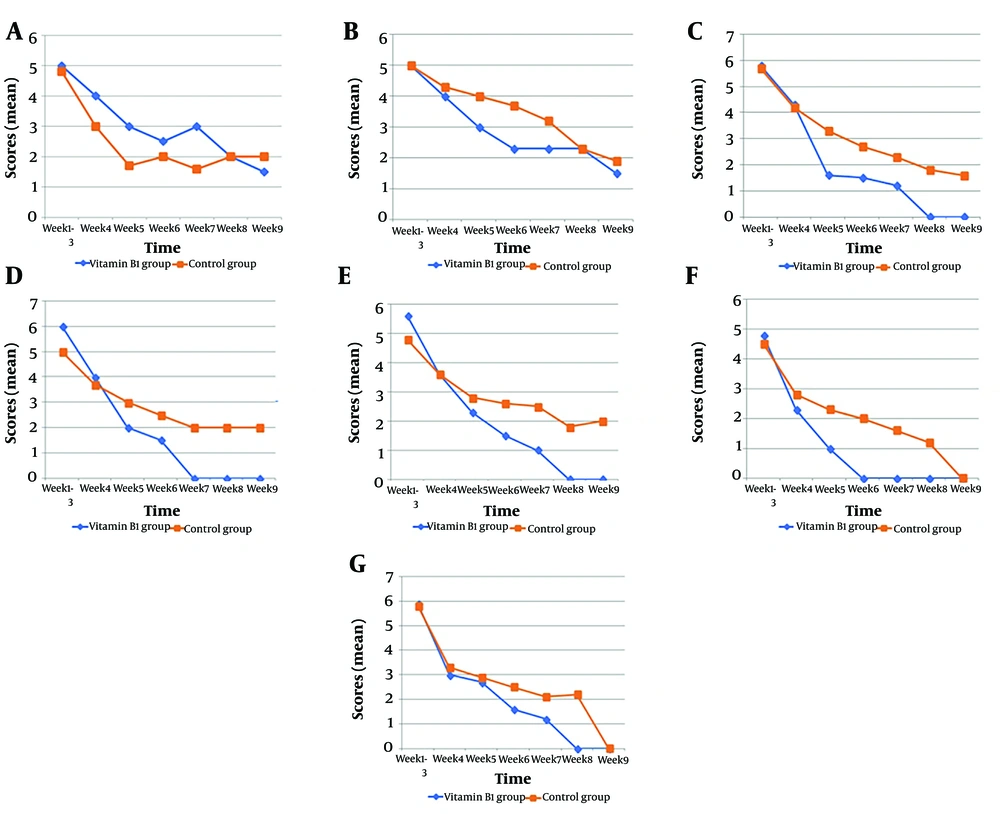
By the seventh week, 67% of symptoms had resolved in the intervention group, compared to only 33% in the control group. In the eighth week, the recovery rate reached 87% in the intervention group, while it was 40% in the control group. After seven weeks, all symptoms in patients receiving vitamin B1 had resolved, except for ageusia, anosmia, anorexia, sleep disturbances, and concentration problems. By the eighth and ninth weeks, only ageusia and anosmia remained in the intervention group (reported in two patients). From the seventh week onward, the recovery rate in the vitamin B1 group was double that of the control group. Figure 2 illustrates the nine-week timeline trend for post-acute COVID-19 symptoms.
5. Discussion
This study investigated the efficacy of vitamin B1 on post-acute COVID-19 symptoms. The findings indicate that vitamin B1 administration significantly reduced the duration of these symptoms.
Several studies have examined the role of vitamins and trace elements, such as ascorbic acid and zinc, in treating COVID-19 patients (13-15). However, the specific role of thiamine (vitamin B1) in post-acute COVID-19 syndrome remains unclear.
Vitamin B1 is an essential precursor for coenzymes involved in glucose metabolism and plays a crucial role in enhancing immune function. It lowers the risk of various diseases, including cardiac beriberi, encephalopathy, and septic shock. Vitamin B1 is also involved in antibody production and T-cell activation, which are essential in combating SARS-CoV-2. A deficiency in vitamin B1 can lead to inadequate antibody responses and more severe symptoms. Additionally, thiamine diphosphate (TDP)-dependent enzymes participate in various metabolic reactions, along with neurotransmitter biosynthesis and antioxidant activity (16). Vitamin B1 acts as an inhibitor of pyruvate dehydrogenase, which helps limit hypoxia and may reduce the need for hospitalization (17). Although limited, some studies suggest the benefits of vitamin B1 for COVID-19 patients (18). For example, Al Sulaiman et al. showed that supportive treatment with vitamin B1 significantly reduced ICU stay length and mortality rates (19).
Another study indicated that vitamin B1 deficiency could result in encephalopathy in severe COVID-19 patients. Administering intravenous vitamin B1 led to notable neurological improvements within two to five days, which facilitated patient weaning from mechanical ventilation and reduced systemic complications, such as ventilator-associated pneumonia and recurrent sepsis (20). Vatsalya et al. also found that vitamin B1 has therapeutic effects in mitigating the pro-inflammatory response of Th17 cells, which may aid in cytokine storm management (21).
A common consequence of COVID-19 is reduced appetite, potentially leading to malnutrition (22). Vitamin B1 deficiency is a significant factor in malnutrition among critically ill patients. Hypovitaminosis of vitamin B1 impairs ATP production, oxygen utilization, and can lead to cardiovascular issues, heart failure, and, if left untreated, death (23). In our study, we observed that vitamin B1 administration increased patient appetite and energy levels, helping alleviate fatigue and dyspnea. However, our study had limitations, as it relied on subjective patient reports, which may have led to reporting bias. Additionally, we were unable to control for confounding variables, including the interval from symptom onset, pre-intervention inflammatory marker levels, and the severity of patients’ initial COVID-19. Future studies with larger sample sizes are recommended to further validate these findings.
5.1. Conclusions
Our study findings indicate that administering vitamin B1 can significantly reduce the duration of post-acute COVID-19 syndrome. Additionally, for patients experiencing post-acute COVID-19 symptoms, supplementing with vitamin B1 alongside appropriate supportive therapy is recommended to enhance recovery rates.