1. Background
A few months after the first description of COVID-19 in China, evidence emerged that the situation was escalating worldwide and threatening to become a pandemi (1). Many studies have been published globally on the impact of SARS-CoV-2 infection in children (2). Generally, the disease presents mild symptoms in children; however, patients with complex medical conditions or those from certain racial and ethnic minority groups require more attention due to their increased risk of severe illness (3).
COVID-19 is primarily a viral infection that usually leads to mild symptoms in children. Secondary bacterial infections following COVID-19 are uncommon, especially among outpatients, and children with this infection would not typically be expected to receive antibiotics (4). This concern became more pronounced when it was found that Azithromycin, initially proposed as a drug with potential antiviral properties and reported to have high consumption rates, did not significantly affect the treatment of the disease (5).
Given that severe and acute manifestations may resemble toxic shock syndrome in hospitalized children, existing consensus documents suggest that empirical broad-spectrum antibiotic treatment is appropriate if bacterial infections cannot be ruled out (6, 7). However, there are growing concerns about the potential negative impacts of the pandemic on children's health and education, particularly regarding the overuse of antibiotics. While this issue is especially contentious for adults with COVID-19, Velasco-Arnaiz et al. reported preliminary data suggesting that the pandemic could significantly impact the use of antimicrobial drugs in the pediatric inpatient population (8). They evaluated antibiotic prescriptions during and before the epidemic but did not assess the direct use of antibiotics and their determinants in children, particularly in outpatient COVID-19 cases (9).
As multiple cases continue to be reported worldwide, SARS-CoV-2 is expected to circulate for an extended period. Therefore, the appropriate management of children with COVID-19 remains a priority. The pandemic has not only directly affected children but has also raised concerns about inappropriate prescriptions that could exacerbate the critical issue of antibiotic overuse and antimicrobial resistance (10).
Many countries have experienced multiple waves of coronavirus outbreaks. Empirical data indicate that characteristics varied between waves in terms of clinical symptoms, complications, hospitalization needs, mortality rates, and transmission rates. Governments and health authorities, including the World Health Organization, have continued to educate the public about preventive measures to reduce virus spread, including quarantine protocols (11).
According to recent reports on COVID-19 in Iran, children infected with the virus range from less than four months to 15 years old, with most reported cases being male. Mortality within this age group is rare. Secondary infections and antibiotic use are not mentioned in this study (12). Bacterial co-infections or secondary infections have been reported in only 7 - 8% of patients with COVID-19. However, antibiotic prescribing rates across various studies were estimated at 56.6% to 74.6%, although these rates are lower in children than in adults (38.5% vs. 83.4%) (13). The overuse of antibiotics among hospitalized patients with COVID-19 has been evident due to disease severity and confusion with bacterial infections (14).
To date, no longitudinal studies have focused on the frequency of COVID-19 infections or on antibiotic use due to secondary bacterial infections in pediatric outpatients.
2. Objectives
Given the gap in existing studies, we conducted a longitudinal study in an area with relatively high antibiotic use to primarily evaluate ambulatory antibiotic use in children with COVID-19 and understand the determinants of its use (15).
3. Methods
We conducted a prospective cohort study on patients aged < 15 years visiting health centers in Kerman province, located in southeastern Iran. From February 2020 to August 2022, children diagnosed with SARS-CoV-2 infection by polymerase chain reaction (PCR) and/or the presence of clinical symptoms of COVID-19, along with one or more family members in the same condition, were included in the study. This study was carried out at three outpatient centers for pediatric infectious diseases affiliated with Kerman University of Medical Sciences in southeastern Iran.
To estimate the sample size for this study, we considered several key factors. We reviewed existing literature to determine the expected prevalence of COVID-19 among children in the target population. A confidence level of 95% was chosen to ensure reliable results, and a margin of error of 5% was accepted, which is standard in clinical studies. Using these parameters, we calculated the minimum sample size required to achieve statistical significance. The final sample included all eligible patients who consented to participate during the study period.
The treatment provided was supportive, including antipyretics, antitussives, and vitamin D or other supplements. The need for hospitalization was determined based on the patient's condition, considering the criteria for moderate to severe disease. The prescription of antibiotics during each visit was based on the suspected secondary bacterial infections, such as acute sinusitis and middle ear infections, diagnosed using clinical criteria by a pediatrician or a pediatric infectious disease subspecialist. Data on the number of times a child was diagnosed with COVID-19 were obtained from electronic records.
Additionally, children's symptoms during the five waves of COVID-19 in Iran were investigated for each peak, with the dominant strain identified through a reference laboratory. All clinical characteristics at the time of referral—such as type of treatment, antibiotic administration, and need for hospitalization—were compared across different peaks of the disease. Parents were provided with a brief description of the study and informed that all responses were voluntary, anonymous, and confidential. The Kerman University of Medical Sciences ethics committee reviewed the study's draft and approved it under code IR.KMU.AH.REC.1400.384.
During the study period, data collection forms were completed for children whose parents signed consent forms to participate in the research. Study variables included age, gender, reason for referral, clinical signs and symptoms, duration of symptoms, number of COVID-19 infections, antibiotic use and its cause, and need for hospitalization. Unclear data items at the time of the child's visit were later extracted from electronic files or obtained by contacting parents directly. At the end of the study, the collected data were entered into SPSS 22 for statistical analysis. Descriptive findings were summarized as frequencies and percentages. Median and interquartile ranges were used for continuous variables. Pearson chi-Square tests were used to compare variables across different waves. In all comparative cases, a P-value < 0.05 indicated statistical significance.
4. Results
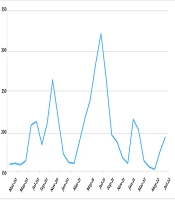
Study Population and Infections: A total of 2,448 children diagnosed with COVID-19 between February 2020 and August 2022 were included in this study. Figure 1 illustrates the monthly distribution of patients, with 1,590 (65%) being male. During the study period, 192 (7.84%) children experienced multiple COVID-19 infections: 35 (1.43%) had three infections, 7 (0.29%) had four infections, and 2 (0.14%) had five infections. Notably, no significant differences were found in underlying diseases, age, gender, or kindergarten attendance between children with single and multiple infections (P = 0.982).
Infection Sources and Hospitalization Rates: Parents were identified as the most frequent source of infection, accounting for 716 cases (29.25%). Among the total study population, 143 children (5.84%) required hospitalization based on clinical assessment, with notably higher rates during the third wave, which coincided with the delta strain (P = 0.007).
Antibiotic Use and Prescribing Patterns: Antibiotics were prescribed to 17.73% (n = 434) of patients, and the rationale for the prescription was unknown in 79.26% of cases. Acute bacterial sinusitis (12.21%) and middle ear infections (8.52%) were the most common reasons for antibiotic prescriptions. Azithromycin (47.70%) and Amoxicillin (29.03%) were the most frequently prescribed antibiotics, followed by Cefuroxime (17.28%) and Co-trimoxazole (5.99%). Notably, the use of Azithromycin significantly declined in subsequent waves compared to the first wave (P = 0.001).
Demographic Characteristics and Symptoms: Table 1 illustrates the demographic characteristics of the children included in the study, while Figure 2 shows the age distribution, indicating an average age of 44.07 ± 34.46 months, with a range from 6 days to 15 years. The most commonly reported symptoms were fever (69.2%) and a decreased sense of smell (0.24%). On average, patients sought medical attention and received diagnoses approximately 3.45 ± 1.72 days after symptom onset, with a range of 1 to 10 days. Table 2 outlines the frequency of clinical signs and symptoms. Although certain gastrointestinal symptoms were more prevalent during the delta wave and laryngitis and hoarseness were more common during the Omicron wave, these variations were not statistically significant (P = 0.266).
| Variables | Wave 1 (n = 337) | Wave 2 (n = 612) | Wave 3 (n = 918) | Wave 4 (n = 367) | Wave 5 (n = 214) | P-Value b |
|---|---|---|---|---|---|---|
| Index case | 0.982 | |||||
| Parents | 99 (29.37) | 179 (29.24) | 268 (29.19) | 107 (29.15) | 63 (29.43) | |
| Other families | 16 (4.74) | 30 (4.90) | 45 (4.90) | 18 (4.90) | 0 (4.67) | |
| Kindergarten | 32 (9.49) | 58 (9.47) | 86 (9.36) | 35 (9.53) | 20 (9.34) | |
| Unknown | 190 (56.37) | 346 (56.53) | 518 (56.42) | 207 (56.40) | 121 (56.54) | |
| Management | 0.007 | |||||
| Outpatient | 325 (96.43) | 584 (95.42) | 844 (91.93) | 348 (94.82) | 204 (95.32) | |
| Hospitalization | 12 (3.56) | 28 (4.57) | 74 (8.06) | 19 (5.17) | 10 (4.67) | |
| Antibiotics | ||||||
| Azithromycin | 0.001 | |||||
| Yes | 37 (10.98) | 49 (8) | 75 (8.16) | 29 (7.90) | 0 | |
| No | 300 (89.02) | 563 (91.99) | 843 (91.83) | 338 (92.09) | 214 (100) | |
| Co-cotrimoxazole | 0.964 | |||||
| Yes | 5 (1.48) | 6 (0.98) | 10 (1.08) | 4 (1.08) | 2 (0.93) | |
| No | 332 (98.51) | 606 (99.01) | 917 (99.89) | 363 (98.91) | 212 (99.06) | |
| Amoxicillin | 0.982 | |||||
| Yes | 17 (5.04) | 32 (5.22) | 47 (5.11) | 19 (5.17) | 11 (5.14) | |
| No | 320 (94.95) | 580 (94.77) | 871 (94.88) | 348 (94.82) | 203 (94.85) | |
| Cefuroxime | 0.903 | |||||
| Yes | 10 (2.96) | 19 (3.10) | 28 (3.05) | 11 (2.99) | 6 (2.80) | |
| No | 327 (97.03) | 593 (96.89) | 890 (96.94) | 356 (97) | 208 (97.19) |
a Values are expressed as No. (%).
b P < 0.05 was considered statistically significant.
| Variables | Wave 1 (n = 337) | Wave 2 (n = 612) | Wave 3 (n = 918) | Wave 4 (n = 367) | Wave 5 (n = 214) | P-Value |
|---|---|---|---|---|---|---|
| Fever | 0.87 | |||||
| Yes | 223 (66.17) | 424 (69.28) | 635 (69.17) | 254 (69.20) | 148 (69.15) | |
| No | 114 (34.13) | 188 (30.71) | 283 (30.82) | 113 (30.79) | 66 (30.84) | |
| Sore throat | 0.978 | |||||
| Yes | 35 (10.38) | 63 (10.29) | 94 (10.23) | 38 (10.35) | 22 (10.28) | |
| No | 302 (89.61) | 549 (89.7) | 824 (89.76) | 329 (89.64) | 192 (89.71) | |
| Coryza | 0.976 | |||||
| Yes | 74 (21.95) | 134 (21.89) | 200 (21.78) | 80 (21.79) | 47 (21.69) | |
| No | 263 (78.04) | 478 (78.1) | 718 (78.21) | 287 (78.2) | 167 (78.03) | |
| Cough | 0.996 | |||||
| Yes | 129 (38.27) | 235 (38.39) | 351 (38.23) | 141 (38.41) | 82 (38.31) | |
| No | 208 (61.72) | 377 (61.6) | 567 (61.76) | 226 (61.58) | 132 (61.68) | |
| Diarrhea | 0.995 | |||||
| Yes | 27 (8.01) | 50 (8.16) | 75 (8.16) | 30 (8.17) | 17 (7.94) | |
| No | 310 (91.98) | 562 (91.83) | 843 (91.83) | 337 (91.82) | 197 (92.05) | |
| Nausea and vomiting | 0.99 | |||||
| Yes | 34 (10.08) | 61 (9.96) | 95 (10.34) | 37 (10.08) | 21 (9.81) | |
| No | 303 (89.91) | 551 (90.03) | 823 (89.65) | 330 (89.91) | 193 (90.18) | |
| Headache | 0.943 | |||||
| Yes | 15 (4.45) | 28 (4.57) | 41 (4.46) | 16 (4.35) | 10 (4.67) | |
| No | 322 (95.54) | 584 (95.42) | 877 (95.53) | 351 (95.64) | 213 (99.53) | |
| Weakness | 0.997 | |||||
| Yes | 4 (1.18) | 7 (1.14) | 12 (1.30) | 5 (1.36) | 3 (1.40) | |
| No | 333 (98.81) | 605 (98.85) | 906 (98.69) | 362 (98.63) | 211 (98.59) | |
| Anorexia | 0.959 | |||||
| Yes | 6 (1.78) | 11 (1.79) | 16 (1.74) | 6 (1.63) | 4 (1.86) | |
| No | 331 (98.21) | 601 (98.2) | 902 (98.25) | 361 (98.36) | 210 (98.13) | |
| Vertigo | 0.996 | |||||
| Yes | 1 (0.29) | 2 (0.32) | 3 (0.32) | 1 (0.27) | 1 (0.46) | |
| No | 336 (99.7) | 610 (99.67) | 915 (99.67) | 366 (99.72) | 213 (99.53) | |
| Skin rash | 0.999 | |||||
| Yes | 5 (1.48) | 8 (1.30) | 12 (1.30) | 5 (1.36) | 3 (1.40) | |
| No | 332 (98.51) | 604 (98.69) | 906 (98.69) | 362 (98.63) | 211 (98.59) | |
| Body ache | 0.982 | |||||
| Yes | 17 (5.04) | 30 (4.90) | 45 (4.90) | 18 (4.90) | 11 (5.14) | |
| No | 320 (94.95) | 582 (95.09) | 873 (95.09) | 349 (95.09) | 203 (94.85) | |
| Abdominal pain | 0.993 | |||||
| Yes | 37 (10.97) | 68 (11.11) | 101 (11) | 40 (10.89) | 24 (11.21) | |
| No | 300 (89.02) | 544 (88.88) | 817 (88.99) | 327 (89.1) | 190 (88.78) | |
| Tonsil exudate | 0.83 | |||||
| Yes | 1 (0.29) | 1 (1.16) | 2 (0.21) | 0 | 0 | |
| No | 336 (99.7) | 611 (99.83) | 916 (99.78) | 367 (100) | 214 (100) | |
| Chills | 0.865 | |||||
| Yes | 13 (3.85) | 25 (4.08) | 37 (4.03) | 15 (4.08) | 9 (4.20) | |
| No | 324 (96.14) | 587 (95.91) | 881 (95.96) | 352 (95.91) | 205 (95.79) | |
| Loss of smell | 0.954 | |||||
| Yes | 1 (0.29) | 1 (1.16) | 2 (0.21) | 1 (0.27) | 1 (0.46) | |
| No | 336 (99.7) | 611 (99.83) | 916 (99.78) | 366 (99.72) | 213 (99.53) | |
| Hoarseness | 0.266 | |||||
| Yes | 15 (4.45) | 26 (4.24) | 40 (4.35) | 24 (6.53) | 7 (3.27) | |
| No | 322 (95.54) | 586 (95.75) | 878 (95.64) | 343 (93.46) | 207 (96.72) |
a Values are expressed as No. (%).
5. Discussion
This study involved 2,448 children diagnosed with COVID-19 over 30 months, coinciding with various disease waves in adults (13). Interestingly, the disease peaks did not correlate with specific seasonal patterns. Although the number of patients referred during the third wave, coinciding with the delta strain, was higher, the difference was not statistically significant. It is worth noting that other studies have also reported an increased impact of the delta and Omicron variants on children (13). The delta strain, in particular, led to more infections and subsequent complications, resulting in higher hospitalization rates for children. This may be attributed to the unique characteristics of the delta variant and increased adherence to preventive measures, immunity from prior infection, and/or vaccination in adults (13).
In terms of gender distribution, 65% of the patients were male, which aligns with previous research conducted in similar regions (14). This gender disparity in favor of boys was also observed in other surveys in Iran and various parts of the world (15). Approximately 8% of children experienced multiple infections, although this usually correlated with underlying conditions or specific causes. It is noteworthy that similar studies examining the frequency of COVID-19 infections in children have not been conducted. While some studies have reported multiple COVID-19 infections in adults, specific reasons for this phenomenon were generally not provided (16).
Parents and family members were the most commonly identified sources of children's infections (17). In many instances, the source of infection remained unclear, but it was highly likely that the initial case came from an adult family member. These adults, while potentially asymptomatic due to vaccination-induced immunity, could still transmit the virus to children (17). The need for hospitalization and the occurrence of severe side effects were notably higher during the third wave, coinciding with the delta strain. During this wave, more children were hospitalized due to complications such as severe diarrhea, vomiting, food intolerance, and uncontrollable fevers. Similar results were observed in other studies (18). Despite the increase in hospitalization cases during this wave, the rates of severe and fatal complications, such as lung involvement, did not significantly differ from those during other peaks.
In total, 434 patients (17.73%) received antibiotics during the study (19-22). In the majority of cases (79.26%), the reasons for prescribing antibiotics were unclear, raising concerns about the potentially irrational use of antibiotics during the COVID-19 pandemic. While antibiotics may be necessary for managing suspected or confirmed bacterial infections in COVID-19 patients, they appear to have been unnecessary in most cases. Interestingly, at the outset of the pandemic, antibiotics were prescribed more frequently to patients. Azithromycin was the most commonly prescribed antibiotic during the early stages of the pandemic, possibly due to its perceived anti-inflammatory, antibacterial, and antiviral properties. However, clinical data later refuted its effectiveness in treating COVID-19 (19-22). The high rate of azithromycin prescriptions in this study was likely due to limited information about its ineffectiveness at the time. The most common reasons for antibiotic use were acute bacterial sinusitis and middle ear infections.
The study found that fever was the most commonly reported symptom, consistent with numerous studies worldwide (23). On average, patients sought medical attention 3.45 days after the onset of symptoms, often driven by high fever and concerns about fever-induced seizures, although no cases of seizures due to fever were reported as the reason for clinic visits. Cases involving seizures or severe, sudden complications were typically taken to the hospital by family members or emergency services. The frequency of other clinical symptoms, including diarrhea, vomiting, coryza, hoarseness, and skin rashes, varied across different waves. Changes in the sense of smell and taste could not be adequately assessed in very young children. Similarly, as in other studies, gastrointestinal symptoms were more prevalent in the delta wave, and laryngitis was more common in the Omicron wave. In other cases, there were no significant differences in the frequency of clinical symptoms across different waves (24).
Indeed, various subtypes of SARS-CoV-2 exhibit distinct clinical behaviors in the pediatric population. Severe complications and hospitalization rates are particularly associated with the Delta and Omicron strains. Additionally, it is noteworthy that individuals, including children, can be infected with COVID-19 multiple times.
In the case of outpatient treatment, antibiotics may not be a useful intervention due to the viral nature of the disease and the absence of clinical evidence supporting the presence of secondary bacterial infections. Their use appears to be limited to specific cases, highlighting the importance of judicious antibiotic prescribing to prevent overuse and the development of antimicrobial resistance.