1. Background
Thyroid hormones, the key regulators of metabolism across all bodily organs, are of paramount importance. Any dysregulation in their production or function can lead to severe health issues, with some cases manifesting as subclinical disorders. Thyroid dysfunction can arise from various factors affecting every level of the hypothalamic-pituitary-thyroid axis (1, 2). Notably, the human immunodeficiency virus (HIV) has been implicated as an infectious agent contributing to the development of endocrine disorders, including thyroid-related conditions (3, 4). Both HIV itself and concurrent opportunistic infections have been identified as primary factors disrupting thyroid axis function. Furthermore, despite the substantial benefits of highly active antiretroviral therapy (HAART) in extending the survival of HIV-infected individuals, it is associated with significant side effects, including damage to the endocrine system (3, 5). While numerous studies have explored the relationship between HIV infection and endocrine disorders, it is worth noting that no such studies have been published in the context of Iran. This underscores the need for further investigation and research in this area.
2. Objectives
This study aimed to fill this research gap by elucidating the correlation between HIV infection, concurrent comorbidities, and thyroid diseases. We conducted our investigation among HIV-infected patients who were referred to Loghman-Hakim Hospital during the period spanning from January 2020 to May 2022.
3. Methods
This study represents a cross-sectional investigation conducted between January 2020 and May 2022 within the HIV clinic at Loghman-Hakim Hospital. Ethical approval for this research was granted by the Medical Ethics Committee of Shahid Beheshti University of Medical Sciences (ethical code: IR.SBMU.MSP.REC.1395.684). The study involved a total of 70 HIV-positive patients with no prior history of endocrine disorders. All participants provided informed consent for their inclusion in the study. Human immunodeficiency virus infection was confirmed in all individuals through two positive third-generation enzyme-linked immunoassay (ELISA) HIV antibody tests, accompanied by a positive Western blot test, or two positive fourth-generation ELISA HIV antibody tests. Demographic data, including weight, height, and waist circumference, were collected during physical examinations. Additionally, patients underwent assessments for blood pressure and pulse rate and a comprehensive examination of the skin and neck, including palpation of the thyroid gland.
Serum samples were collected from the participants between 8 and 9 AM for the evaluation of various thyroid-related parameters, including thyroid-stimulating hormone (TSH), thyroxine (T4), triiodothyronine (T3), free T4, T3 uptake, and anti-thyroid peroxidase (anti-TPO) antibody levels. triiodothyronine and T4 measurements were conducted using the ELISA (Euroimmun® kit) and enzyme-linked fluorescent assay (Vidas® ELFA kit) methods. Free T4, T3 uptake, and anti-TPO antibody levels were assessed using the Euroimmun® ELISA kit, while TSH levels were estimated using the Vidas® ELFA kit.
Thyroid axis dysfunctions were categorized based on normal laboratory values:
(1) Subclinical hypothyroidism: Normal T3, T4, free T4, and elevated TSH levels.
(2) Primary hypothyroidism: Low T3, T4, free T4 levels, and TSH levels exceeding 10 µU/L.
(3) Secondary hypothyroidism: Low T3, T4, free T4 levels, and TSH levels ranging between 5.6 µU/L and 10 µU/L.
(4) Thyroxine-binding globulin deficiency: Characterized by normal TSH and free T4 levels alongside low T4 levels and elevated T3 uptake.
In addition to thyroid-related assessments, CD4+ cell levels were measured using CD flow cytometry. The diagnosis of acquired immunodeficiency syndrome (AIDS) in our cases adhered to the latest Centers for Disease Control and Prevention (CDC) surveillance criteria for HIV patients (6). Following the comprehensive data collection encompassing historical information, physical examinations, medical profiles, and laboratory test results, a thorough analysis of the gathered dataset was conducted using SPSS® software (version 23). In the pursuit of identifying potential relationships between two independent categorical variables, the chi-square test was employed. Furthermore, logistic regression tests were conducted to scrutinize the associations between continuous independent and dichotomous variables. Pearson's correlation coefficient analysis assessed the correlation between continuous variables. A P-value less than 0.05 was regarded as the statistical threshold for significance throughout the analysis.
4. Results
This study involved a total of 70 HIV-positive patients, comprising 45 (64.7%) males and 25 (35.3%) females. The average age of the patients was 39.85 years, with a standard deviation of ± 10.54 years, ranging from 21 to 74 years. According to the criteria established by the CDC, 30 patients (42.85%) met the definition of having AIDS. Within this subgroup, 28 (93.33%) patients exhibited a CD4+ cell count of less than 200/ mm³. Among these individuals, three were diagnosed with Pneumocystis jirovecii pneumonia (PCP), while one patient had cerebral toxoplasmosis. The average duration of HIV infection from the time of diagnosis to inclusion in this study was found to be 18.32 months, with a standard deviation of ± 16.18 months.
The most frequently observed concomitant infections among the study participants were hepatitis C, affecting 17.14% of the patients, followed by hepatitis B at 5.7%, and latent tuberculosis also at 5.7%. In terms of pharmaceutical treatments, the most commonly administered drugs, aside from the HAART regimen, included cotrimoxazole, used by 24.2% of the patients, azithromycin by 10%, and isoniazid by 8.6%. Notably, all but three patients in the study received HAART treatment following the ATRIPLA protocol, consisting of a combination of 200 mg emtricitabine, 600 mg efavirenz, and 300 mg tenofovir fumarate.
Additionally, 24.28% of the patients were identified as cigarette smokers, 14.2% were found to be opioid/methamphetamine abusers, and 1.4% reported alcohol consumption as part of their habits. Table 1 summarizes the patients’ characteristic information.
| Variables | No. (%) | Mean ± SD |
|---|---|---|
| Gender (male/female) | 45/25 | - |
| Age (y) | 70 | 39.85 ± 10.54 |
| Duration of infection since HIV diagnosis (mon) | 70 | 18.32 ± 16.18 |
| Confirmed AIDS status | 30 (42.85) | - |
| Active smoker | 16 (22.8) | - |
| BMI | 70 | 25.41 ± 4.69 |
| Treatment with ART | 67 (95.71) | - |
Abbreviations: HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; BMI, Body Mass Index.
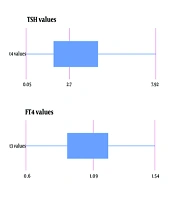
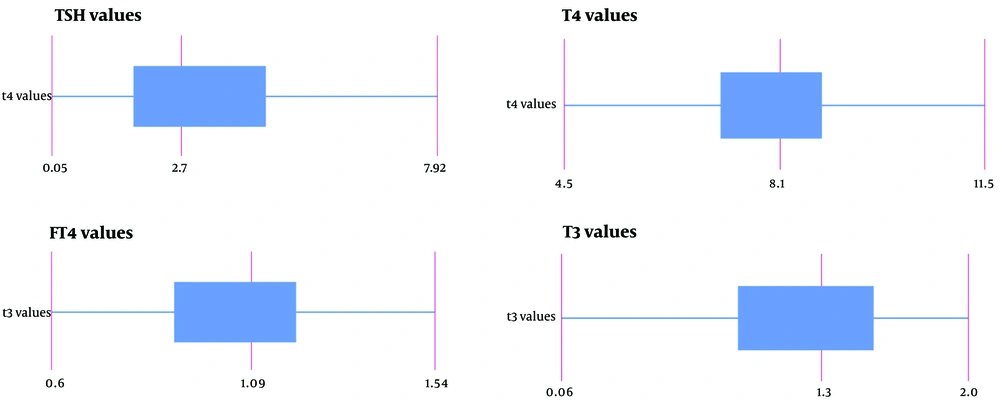
The mean levels of T4, free T4, and T3 were determined to be 8 mcg/dL (with a standard deviation of ± 1.5 mcg/dL), 1.08 ng/dL (± 0.22 ng/dL SD), and 1.26 nmol/L (± 0.42 nmol/L SD), respectively. The average TSH level measured was 4.22 mIU/L (± 7.25 mIU/L SD). Figure 1 demonstrates the boxplot of thyroid function test values.
Based on the definitions used to assess the hypothalamic-pituitary-thyroid axis function, thyroid dysfunction was identified in 27.1% of the patients. Notably, thyroid dysregulation was more common in females than in males, with rates of 36% and 22.2%, respectively. Among the various thyroid conditions observed, subclinical hypothyroidism was the most prevalent, affecting 15.7% of the patients. Primary hypothyroidism accounted for 4% of the cases, while another 4% had isolated elevated anti-TPO antibody levels with normal thyroid function. Additionally, 1.4% of the patients were found to have primary hyperparathyroidism, and another 1.4% were diagnosed with subclinical hyperparathyroidism. Table 2 illustrates the thyroid function status.
| Thyroid Function Status | No. (%) |
|---|---|
| Normal | 51 (72.8) |
| Subclinical hypothyroidism | 11 (15.7) |
| Primary hypothyroidism | 3 (4) |
| Evidence of autoimmunity without thyroid dysfunction | 3 (4) |
| Subclinical hyperthyroidism | 1 (1.4) |
| Primary hyperthyroidism | 1 (1.4) |
The chi-square test identified no statistically significant relationships between gender, AIDS status, and the ATRIPLA protocol, as indicated by P-values exceeding 0.05. Subsequently, logistic regression tests were conducted to evaluate the association between age, duration of HIV infection, CD4+ cell count, and the hypothalamic-pituitary-thyroid axis. The results revealed no statistically significant association between these variables and thyroid dysfunction, with P-values exceeding 0.05. Furthermore, when examining the mean levels of TSH, T4, free T4, and T3, there were no statistically significant differences observed between AIDS and non-AIDS patients, further reinforcing the absence of significant associations in this context (Table 3).
| Variables | OR (95% CI) | P-Value |
|---|---|---|
| Age | 0.978 (0.924 - 1.035) | 0.438 |
| Sex | - | 0.343 |
| Positive AIDS status | - | 0.558 |
| Interval from HIV diagnosis | 0.992 (0.958 - 1.027) | 0.635 |
| ATRIPLA | - | 0.288 |
| CD4+ level | 1.000 (0.999 - 1.002) | 0.626 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
5. Discussion
The improved survival of HIV-positive patients, largely attributed to the widespread availability of antiretroviral therapies and enhanced medical access on a global scale, has shifted attention toward addressing long-term morbidity concerns, including endocrine disorders (5, 7-11). Among these, the hypothalamus-pituitary-thyroid axis, which plays a pivotal role in the metabolism of virtually every organ, has garnered significant interest due to its susceptibility to impairment during HIV infection through various mechanisms (12, 13).
Several mechanisms have been proposed to explain the pathogenesis of thyroid dysfunction in patients living with HIV. Opportunistic and concurrent infections, besides HIV itself, may activate immune responses, leading to chronic inflammation (14). This inflammation can interfere with thyroid gland functions. Additionally, HIV has a direct cytotoxic effect on glandular tissues, including the thyroid gland. It has been shown that some HAART agents, such as efavirenz and stavudine, may cause thyroid dysfunction by probable interference with thyroid hormone metabolism (15, 16). The prevalence of thyroid gland dysfunction varies across studies and geographical regions (16-18).
This study was conducted at a single medical center and aimed to shed light on the prevalence of thyroid diseases among HIV-positive patients. Importantly, it marks the first investigation of endocrinopathies in HIV patients carried out in Iran. The findings revealed that 26.5% of the patients exhibited abnormalities within the thyroid axis, with subclinical hypothyroidism emerging as the most frequently detected condition. Crucially, this study did not identify any associations between CD4+ cell levels, AIDS status, infection duration, and gender in thyroid dysfunction among HIV-positive individuals. These findings underscore the importance of monitoring and addressing endocrine disorders as part of comprehensive care for HIV-infected patients, contributing to their overall quality of life.
The study conducted by Tripathy et al. focused on 43 Indian patients living with HIV. Their findings indicated that 60.4% of these patients were diagnosed with thyroid dysfunction. The three most prevalent thyroid conditions observed in this study were isolated low T3, secondary hypothyroidism, and subclinical hypothyroidism, in descending order of frequency. Interestingly, their research did not reveal any correlation between CD4+ cell levels and thyroid function in HIV-positive individuals (19).
In a separate case-control study conducted in Nigeria by Emokpae and Akinnuoye, which involved 200 HIV-positive cases and 100 healthy individuals in the control group, a thyroid function abnormality incidence of 52% to 56% was reported. The sick euthyroid syndrome was identified as the most common abnormality, observed both in patients receiving HAART and those who were HAART-naive. Notably, this study observed lower levels of TSH, T4, and T3 in HIV patients compared to the control group. Interestingly, in contrast to the findings of the study discussed earlier, Emokpae and Akinnuoye discovered a positive correlation between CD4+ cell levels and T4 levels in HIV-positive patients (20).
These studies collectively underscore the variability in the prevalence and characteristics of thyroid dysfunction among HIV-positive individuals, which can be influenced by factors such as geographic location, patient demographics, and treatment regimens. Furthermore, the relationship between CD4+ cell levels and thyroid function appears to be a subject of ongoing investigation with varying findings across different studies (21). Contrastingly, some extensive population studies have failed to demonstrate a significant alteration in the prevalence of thyroid dysfunction among HIV-positive patients when compared to control groups (15).
In a retrospective study conducted in the United States by Gallant et al., the evaluation of comorbidities in individuals diagnosed with HIV between 2003 and 2013 revealed that the estimated prevalence of thyroid diseases varied between 6.1% to 20.4% among three subgroups, categorized based on their medical payers. The subgroup with the highest rate of thyroid disease also had a notably higher mean age (42 years versus 71 years) (22).
A retrospective cohort study involving 6,343 Italian HIV-positive patients spanning from 2005 to 2017 reported a clinical thyroid disease incidence of 1.94%. Among these patients, hypothyroidism was the most prevalent thyroid disorder, accounting for 66% of cases, followed by hyperthyroidism at 17%, primitive tumors at 9%, and simple goiter at 8%. Hashimoto thyroiditis (78%) and graves' disease (17%) were identified as the primary causes of hypothyroidism and hyperthyroidism, respectively. Interestingly, most patients in this study were well-treated for their HIV infection. The study also found that male gender was associated with a protective effect against hypothyroidism, while age was linked to the development of both hypothyroidism and hyperthyroidism. Additionally, CD4+ cell levels below 200 cells/mm³ were associated with an increased risk of hyperthyroidism (23).
Similarly, a study by Harslof et al. in Denmark among well-treated HIV-positive patients observed a prevalence of hypothyroidism (3.8%) and hyperthyroidism (0.8%) that was nearly identical to a matched non-infected control group (4.6% and 0.8%, respectively) (24). These findings highlight the variability in results across different population studies and suggest that the prevalence of thyroid dysfunction in HIV-positive individuals may be influenced by factors such as geographic location, the population studied, and the extent of HIV treatment and control.
While our study represents a valuable contribution as the first of its kind in our country, it is essential to acknowledge its inherent limitations. One of the primary constraints was the absence of access to multicenter databases, limiting the diversity and size of our study population. This limited sample size could potentially influence the generalizability and robustness of our findings. Another noteworthy limitation was the absence of a control group in our study, which would have allowed us to make direct comparisons with the thyroid health of the general population. The absence of a control group makes it challenging to discern whether the observed thyroid dysfunction is specifically associated with HIV infection or is reflective of broader trends in the population.
5.1. Limitations
In light of these limitations, we strongly recommend conducting larger-scale studies, particularly those involving diverse populations, and incorporating control groups to enable more comprehensive and reliable results, especially in the context of developing countries. These expanded studies can provide a more thorough understanding of the relationship between HIV infection and thyroid dysfunction, helping to inform clinical management and improve the quality of care for HIV-positive individuals.
5.2. Conclusions
In conclusion, the incidence of thyroid abnormalities among people living with HIV remains a topic of debate and variability across different societies. However, emerging evidence suggests that factors such as age, gender, and the quality of treatment may play significant roles in the development of thyroid dysfunction in this population. To gain a clearer understanding of the specific risk and protective factors associated with thyroid diseases in individuals living with HIV, further research is imperative. These studies should encompass diverse populations and consider regional variations. The insights gained from such investigations will not only contribute to a more comprehensive understanding of thyroid dysfunction in HIV-positive individuals but will also aid in refining healthcare strategies and improving the overall health status of this patient group.