1. Background
On December 31, 2019, excessive viral respiratory infections, namely COVID-19, were reported in Wuhan, China (1), and rapidly spread worldwide. The causative virus of COVID-19 is SARS-CoV-2, which belongs to the Coronaviridae family and is a high-quality single-stranded RNA virus surrounded by an envelope.
Recent studies have highlighted the severe consequences of COVID-19, which involve different systems in the body. One of the most significant findings is the presence of SARS-CoV-2 RNA in heart tissue and the involvement of the cardiovascular system during the disease (2). However, there is no evidence of direct cardiotropic cell infection. Atherosclerosis and other cardiovascular disorders are risk factors during COVID-19 development (2). These disorders pose a threat to COVID-19 outcomes, and conversely, COVID-19 can result in cardiovascular issues, including myocarditis, myocardial damage, and myocardial infarction. Although COVID-19, unlike atherosclerosis, is an infectious disease, inflammation and the immune response are crucial components in the pathogenesis of both conditions (3).
All viruses of the Coronaviridae family require host cells for viral replication, necessitating an increase in the host cell's metabolism to cope with viral replication (4). Lipid metabolism is also affected in viral infections (5). Viruses must regulate the lipid synthesis of the host cell to produce the lipids required for their envelopes (6, 7). Cholesterol and fatty acids are essential components of viral membranes and are necessary for viral replication (8). Previous studies, particularly on RNA viruses, show the effects of these viruses on lipid metabolism. For example, it has been demonstrated that LDL-C levels decrease and HDL-C levels increase in patients with HIV (9). Additionally, triglyceride (TG) levels increased in the advanced stages of the disease (10). Triglycerides and HDL-C, which assess the risk of cardiovascular mortality, are two lipids routinely measured (11). It is well known that optimal levels of blood lipid factors are essential in preventing heart diseases. Total cholesterol to high-density lipoprotein (HDL), and low-density lipoprotein (LDL) to HDL ratios are two important factors in blood fat related to atherosclerosis. Total cholesterol/HDL below 3.5 and LDL/HDL below 4.5 are considered safe for heart diseases (12).
Considering SARS-CoV-2 infection, binding through angiotensin-converting enzyme 2 (ACE2) leads to the reduction of membrane-bound ACE2 and the simultaneous loss of ACE2 catalytic activity in the RAS system, causing a decrease in the level of Ang-(1-7) and an increase in Ang-II. Unlike ACE2/Ang-(1-7), Ang-II can promote inflammation and oxidative stress, contributing to the development of atherosclerosis (13). SARS-CoV-2 attacks host cells through two receptors: Angiotensin-converting enzyme 2 and CD147. The spike protein (SP) of the virus binds to ACE2 or CD147 on the host cell, mediating viral invasion and virus release (14). The structure of SARS-CoV-2 SP is similar to SARS-CoV SP, and both bind to ACE2 to attack host cells. In addition to ACE2, Wang et al. recently showed that the SARS-CoV-2 spike protein also binds to CD147 (16). Studies indicate that CD147 plays a functional role in facilitating SARS-CoV-2 infection, and a CD147 antagonist peptide-9 has been found to inhibit SARS-CoV-2. These findings confirm the importance of CD147 in the viral infection of host cells. The direct interaction of CD147 and the SARS-CoV-2 spike protein has been reported to mediate virus infection in host cells. Studies have also highlighted the potential pro-atherosclerotic effects of CD147 (15).
In some patients with COVID-19, increased levels of factors such as creatine kinase (CPK) and CRP are reported (16). It has been proven that the levels of acute-phase inflammatory markers such as CRP, lactate dehydrogenase (LDH), and troponin are higher in COVID-19 patients. Myocardial injury is often observed among patients with COVID-19, frequently indicated by elevated troponin levels (17).
2. Objectives
This study aimed to investigate the activity of ACE, ACE2, CD147, and troponin, as well as the concentrations of cholesterol, triglycerides, HDL, and LDL, to determine atherosclerosis risk factors in patients with severe and non-severe COVID-19.
3. Material
3.1. Sample Collection and Section
A total of 90 subjects (48 males and 42 females) with a mean age of 47.21 years were enrolled in this case-control study. The study included thirty patients in the ICU (severe), thirty hospitalized patients (non-severe), and thirty control subjects (negative for COVID-19) who were admitted to Ayatollah Kashani Hospital (Tehran, Iran). The sample size was calculated using the formula N = (Z 1-α/2 + Z 1-β)2 × (δ12 + δ22) / (µ1 - µ2)2, considering a statistical power of 90% (Z1-β) and a 95% confidence level (Z1-α/2). Based on the flow chart for the diagnosis and treatment of COVID-19, patients were divided into outpatient and inpatient service levels according to the protocol of the Ministry of Health of Iran. Pharyngeal swab specimens were collected from all participants for SARS-CoV-2 testing, and PCR (SANSURE Novel Coronavirus Nucleic Acid Diagnostic Kit) was performed to determine the presence (inpatients) or absence of infection (in controls). The study protocol was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (ethical code: IR.SBMU.RETECH.REC.1401.355).
3.2. Determination of Cholesterol Concentration
Cholesterol levels were measured using the enzyme method and the Byrex Fars kit (Iran). In this method, cholesterol esters are hydrolyzed by the cholesterol esterase enzyme, and the resulting cholesterol reacts with cholesterol oxidase to produce hydrogen peroxide. The hydrogen peroxide then reacts with phenol and 4-aminoantipyrine to produce a red quinone compound. The intensity of the color formed is directly proportional to the cholesterol level in the sample, which is reported as mg/dL.
3.3. Determination of Triglyceride Concentration
Triglycerides were measured using the enzyme method and the Byrex Fars kit (Iran). In this method, glycerol is first separated from fatty acids by the enzyme lipoprotein lipase. Hydrogen peroxide is then released from glycerol through a reaction with 4-aminoantipyrine and phenol in the presence of the peroxidase enzyme, forming quinoneimine. The amount of quinoneimine produced is directly proportional to the concentration of triglycerides and is reported as mg/dL.
3.4. Determination of High-Density Lipoprotein Concentration
The reaction is based on accelerating the reaction of cholesterol oxidase with non-esterified HDL cholesterol and dissolving HDL using detergents. Using the Pars Azmoun kit (Iran), the esterified HDL cholesterol undergoes an enzymatic reaction, and the peroxidase resulting from this reaction, together with DSBMT (N-bis 4-sulphobutyl-m-toluidine), produces a colorless product. Finally, it reacts with cholesterol esterase and is reported as mg/dL.
3.5. Determination of Low-Density Lipoprotein Concentration
Using the Pars Azmoun kit (Iran), all lipoproteins other than LDL, including HDL, VLDL, and chylomicrons, were removed. The concentration of LDL cholesterol was then specifically calculated using a colorimetric enzyme reaction and reported as mg/dL.
3.6. Measurement of Angiotensin-Converting Enzyme Activity
Angiotensin-converting enzymein the sample causes the hydrolysis of the FAPGG (Furylacryloyl-phenylalanyl-glycyl-glycine) substrate, resulting in the formation of FAP (Furylacryloyl-phenylalanyl). This procedure was performed using the Biorex Fars (Iran) kit. The decrease in absorbance, measured at a wavelength of 742 nm, is proportional to the amount of ACE in the sample and is reported as U/L.
3.7. Measurement of Angiotensin-Converting Enzyme 2 Enzyme Activity
The measurement was done using an ELISA kit (MyBioSource, USA). This method is based on Sandwich-ELISA, and the micro-ELISA plate provided in this kit is already coated with a specific human antibody for ACE2 (MyBioSource, USA). The absorbance was read at a wavelength of 450 ± 2 nm. The optical density (OD) value is proportional to the concentration of human ACE2 and is reported as U/L.
3.8. Measurement of Serum CD-147 Level
This work was performed using a Thermo Fisher kit (USA). The immunosorbent assay for CD147 uses a quantitative sandwich immunoassay technique. The microtiter plate provided in this kit is pre-coated with a CD147 monoclonal antibody (Thermo Fisher, USA). The color change was measured spectrophotometrically at a wavelength of 450 nm and determined as pg/mL.
3.9. Measurement of Serum Troponin Enzyme Activity
The troponin level was measured using the Biorex Fars kit (Iran) with the turbidometry method and a photometer and reported as ng/mL.
3.10. Measurement of Serum CPK and CPK-mb (Myocardial band) Enzyme Activity
The measurement was performed using the Byrex Fars kit (Iran). Creatine kinase catalyzes the transfer of the phosphate group from phosphocreatine to ADP (adenosine diphosphate). This reaction is coupled with reactions catalyzed by hexokinase and G6PDH (glucose-6-phosphate dehydrogenase). The rate of NADH formation was measured photometrically and is proportional to the catalytic concentration of creatine kinase in the sample, reported as U/L.
3.11. Statistical Analysis
All statistical analyses were performed using IBM SPSS V21 software. The Mann-Whitney and t-test were used to compare two unpaired groups, while the Kruskal-Wallis test was used to compare three groups. The results are presented with significance levels of P < 0.05 and P < 0.01.
4. Results
4.1. Patients Characteristics
The participants in this study comprised 30 (33%) ICU (severe) COVID-19 patients, 30 (33%) ward-admitted (non-severe) COVID-19 patients, and 30 (33%) healthy control (negative COVID-19) cases. The total sample included 54 (60%) men and 36 (40%) women, with a mean age of 61.33, 50.6, and 57.1 in the ICU (severe), ward (non-severe), and control groups, respectively.
4.2. Comparison of Studied Parameters Between the Patients and Control Groups
The results showed significant differences among the three groups (ICU or severe, ward or non-severe, and control or negative) in terms of age, ACE2, CPK, LDH, LDL, HDL, CPK-mb, triglyceride, and the ratio of cholesterol to HDL (P < 0.05). However, CD-147 (2528.43 ± 12.43 vs. 2176.7 ± 9.87 vs. 1346.3 ± 14.23), ACE (83 ± 3.05 vs. 97 ± 1.55 vs. 30.1 ± 2.32), troponin (0.972 ± 0.25 vs. 0.784 ± 0.21 vs. 0.021 ± 0.68), and cholesterol levels (199.73 ± 2.43 vs. 175.87 ± 5.21 vs. 144.97 ± 8.74) were significantly higher in severe cases compared to non-severe ones (P < 0.05). The mean values and P-values for each group are presented in Table 1.
| Parameters | Severe (ICU) | Non-severe (Ward) | Control (C) | P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min - Max | Mean | Min - Max | Mean | Min - Max | Three Groups | ICU vs. C | Ward vs. C | ICU vs. Ward | |
| Age | 61.33 | 45 - 80 | 50.6 | 33 - 78 | 57.1 | 40 - 78 | 0.002 | 0.162 | 0.033 | 0.001 |
| CD147 | 2528.43 | 1200 - 3461 | 2176.7 | 1100 - 3111.2 | 1346.3 | 1008 - 1652 | 0.000 | 0.000 | 0.000 | 0.010 |
| ACE2 | 51.73 | 17 - 87 | 51.13 | 16 - 87 | 23.47 | 10 - 40 | 0.000 | 0.000 | 0.000 | 0.853 |
| ACE | 83 | 41 - 116 | 55.77 | 12 - 97 | 30.1 | 16 - 52 | 0.000 | 0.000 | 0.000 | 0.000 |
| CPKmb | 32.3667 | 12.6 - 60.2 | 36.46 | 15.6 - 60.4 | 17.5933 | 12.6 - 28.4 | 0.000 | 0.000 | 0.000 | 0.234 |
| CPK | 161.8 | 62 - 301 | 182.3 | 78 - 302 | 87.97 | 63 - 142 | 0.000 | 0.000 | 0.000 | 0.234 |
| Trop | 0.972 | 0.1 - 3.4 | 0.784 | 0.1 - 20 | 0.021 | 0.01 - 0.04 | 0.000 | 0.000 | 0.004 | 0.016 |
| LDH | 191.47 | 103 - 312 | 183.63 | 102 - 302 | 139.4 | 109 - 183 | 0.000 | 0.001 | 0.001 | 0.559 |
| LDL | 83.5 | 23 - 165 | 73.37 | 32 - 110 | 51.27 | 34 - 75 | 0.000 | 0.000 | 0.000 | 0.258 |
| HDL | 44 | 26 - 66 | 45.23 | 26 - 110 | 53.8 | 41 - 79 | 0.001 | 0.005 | 0.000 | 0.906 |
| TG | 180.83 | 86 - 310 | 156.97 | 87 - 269 | 130.7 | 85 - 220 | 0.002 | 0.002 | 0.134 | 0.188 |
| Chol | 199.73 | 126 - 297 | 175.87 | 109 - 297 | 144.97 | 102 - 211 | 0.000 | 0.000 | 0.008 | 0.072 |
| Chol/HDL | 4.99 | 2.14 - 10.69 | 4.201 | 1.05 - 7.15 | 2.74 | 1.55 - 4.25 | 0.000 | 0.000 | 0.000 | 0.315 |
Abbreviations: ACE, angiotensin-converting enzyme (ACE); LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride.
When comparing severe (ICU) and non-severe (ward) cases with the control group, all studied parameters showed a significant difference, except for the age of ICU vs. control and the triglyceride level of ward vs. control. The level of troponin showed no significant difference among all compared groups.
Further comparison of severe (ICU) with non-severe (ward) cases revealed a significant increase in CD147, ACE, troponin, and cholesterol with increasing disease severity. A significantly higher mean age was observed in severe cases compared to non-severe ones. Conversely, there was a decrease in HDL levels with increasing disease severity. The detailed data are presented in Table 1.
4.3. Assessment of Sensitivities and Specificities of Variables
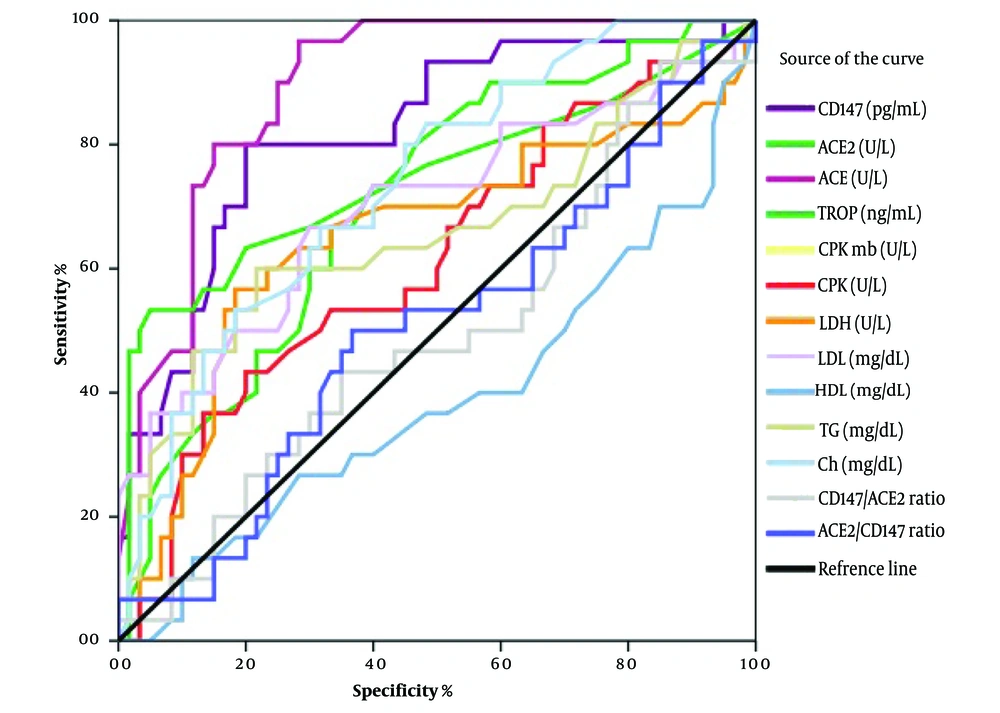
Receiver operating characteristic (ROC) curves were used to predict the sensitivity and specificity of the laboratory tests. The areas under the curves (AUCs) and P-values were as follows: AUC = 0.894, P < 0.001 for ACE activity; AUC = 0.821, P < 0.001 for CD147 activity; AUC = 0.746, P < 0.001 for troponin levels; AUC = 0.736, P < 0.001 for cholesterol concentration; AUC = 0.708, P < 0.001 for ACE2 activity; AUC = 0.696, P < 0.001 for LDL concentration; AUC = 0.657, P = 0.016 for triglyceride concentration; and AUC = 0.652, P = 0.019 for LDH activity. Among these, ACE and CD147 levels demonstrated the highest sensitivity and specificity compared with other variables (Figure 1).
4.4. Assessment of Correlations Among the Studied Parameters
Pearson's correlation coefficient analysis revealed several significant correlations among the studied parameters, as shown in Table 2 R-values over 0.6 were considered strong correlations. The strongest correlations were observed between CPKmb and CPK (r = 1, P = 0.000), ACE2/CD147 ratio and ACE2 (r = 0.721, P = 0.000), CD147 and ACE2 (r = 0.646, P = 0.000), and cholesterol and triglyceride (r = 0.602, P = 0.000). Moderate correlations were also found between ACE level and LDL, CD147, ACE2, and cholesterol levels; LDL level and CPKmb, CPK, and cholesterol levels; CD147 level and CPKmb and CPK; CPKmb level and LDH and HDL; and CPK level and LDH and HDL.
| Variables (R) | ACE | LDL | Age | CD147 | ACE2 | TROP | CPKmb | CPK | LDH | HDL | TG | Chol | ACE2/CD147 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE | 1 | 0.441b | 0.014 | 0.436 b | 0.448 b | -0.018 | 0.336 b | 0.336 b | 0.325 b | -0.195 | 0.292 b | 0.403 b | 0.244 c |
| LDL | 0.441 b | 1 | -0.040 | 0.393 b | 0.319 b | 0.277 b | 0.445 b | 0.445 b | 0.187 | -0.345 b | 0.389 b | 0.445 b | 0.150 |
| Age | 0.014 | -0.040 | 1 | 0.153 | 0.004 | 0.096 | -0.229 c | -0.229 c | -0.107 | 0.069 | 0.039 | 0.008 | -0.141 |
| CD147 | 0.436 b | 0.393 b | 0.153 | 1 | 0.646 b | 0.242 c | 0.416 b | 0.416 b | 0.291 b | -0.252 c | 0.293 b | 0.380 b | -0.014 |
| ACE2 | 0.448 b | 0.319 b | 0.004 | 0.646 b | 1 | -0.072 | 0.316 b | 0.316 b | 0.152 | -0.250 c | 0.235 c | 0.256 c | 0.721 b |
| TROP | -0.018 | 0.277 b | 0.096 | 0.242 c | -0.072 | 1 | 0.066 | 0.066 | 0.130 | -0.141 | 0.149 | 0.296 b | -0.216 c |
| CPKmb | 0.336 b | 0.445 b | -0.229 c | 0.416 b | 0.316 b | 0.066 | 1 | 1.000 b | 0.474 b | -0.413 b | 0.088 | 0.318 b | 0.102 |
| CPK | 0.336 b | 0.445 b | -0.229 c | 0.416 b | 0.316 b | 0.066 | 1.000 b | 1 | 0.474b | -0.413 b | 0.087 | 0.318 b | 0.102 |
| LDH | 0.325 b | 0.187 | -0.107 | 0.291b | 0.152 | 0.130 | 0.474 b | 0.474 b | 1 | -0.054 | -0.016 | 0.218 c | 0.008 |
| HDL | -0.195 | -0.345 b | 0.069 | -0.252 c | -0.250 c | -0.141 | -0.413 b | -0.413b | -0.054 | 1 | -0.184 | -0.264 c | -0.112 |
| TG | 0.292 b | 0.389 b | 0.039 | 0.293 b | 0.235 c | 0.149 | 0.088 | 0.087 | -0.016 | -0.184 | 1 | 0.602 b | 0.054 |
| Chol | 0.403 b | 0.445 b | 0.008 | 0.380 b | 0.256 c | 0.296 b | 0.318 b | 0.318 b | 0.218 c | -0.264 c | 0.602 b | 1 | 0.051 |
| ACE2/CD147 | 0.244 c | 0.150 | -0.141 | -0.014 | 0.721 b | -0.216 c | 0.102 | 0.102 | 0.008 | -0.112 | 0.054 | 0.051 | 1 |
Abbreviations: ACE, angiotensin-converting enzyme (ACE); LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride.
a R-values below 0.2 were interpreted as very weak correlations. R-values between 0.2 and 0.4 were interpreted to be weakly correlated, 0.4 to 0.6 moderately, 0.6 to 0.8 strongly, and 0.8 to 1.0 very strongly correlated. Three strong correlations were found among the studied parameters; CD147 and ACE2/CD147 with ACE2 and cholesterol with triglyceride were strongly correlated.
b Correlation is significant at the 0.01 level (2-tailed).
c Correlation is significant at the 0.05 level (2-tailed).
4.5. Association Study Between Studied Parameters and Disease Severity
Odds ratio analysis was carried out to find the association between the studied parameters and the severity of COVID-19 disease. Patients were categorized into two groups: Those admitted to the hospital ward and those admitted to the ICU. The results showed that patients with normal ACE2, CPKmb, CPK, and HDL levels were 1.167, 4.375, 1.306, and 2.143 times more likely to be admitted to the ICU with severe disease, respectively (Table 3).
| Odds Ratio | Value | 95% Confidence Interval | |
|---|---|---|---|
| Lower | Upper | ||
| CD147 (N/H) | 0.248 | 0.084 | 0.729 |
| ACE2 (N/H) | 1.167 | 0.393 | 3.467 |
| ACE (N/H) | 0.167 | 0.053 | 0.529 |
| Troponin (N/H) | 0.386 | 0.136 | 1.094 |
| CPKmb (N/H) | 4.375 | 1.320 | 14.504 |
| CPK (N/H) | 1.306 | 0.474 | 3.602 |
| LDH (N/H) | 0.507 | 0.180 | 1.422 |
| LDL (N/H) | 0.545 | 0.183 | 1.623 |
| HDL (N/L) | 0.467 | 0.135 | 1.609 |
| Triglyceride (N/H) | 0.327 | 0.104 | 1.032 |
| Cholesterol (N/H) | 0.398 | 0.131 | 1.210 |
Conversely, patients with normal levels of CD147, ACE, Troponin, LDH, LDL, Triglyceride, and Cholesterol had a 0.248, 0.167, 0.386, 0.507, 0.545, 0.327, and 0.398 times lower chance of being admitted to the ICU, respectively. Further analysis showed that the odds ratio was significant only for ACE and CPKmb.
5. Discussion
SARS-CoV-2 is a positive single-strand RNA virus from the Coronaviridae family, which has become a global pandemic. The disease known as COVID-19 is highly contagious and has become a significant health burden due to its widespread and varied mechanisms of pathogenesis. The cardiovascular system can be affected by the virus (2).
Atherosclerosis is a severe and chronic inflammatory disease characterized by thickened artery walls. This increased thickness and plaque accumulation lead to narrowed and tightened channels, raising blood pressure. Several factors contribute to the development of this disease, including high cholesterol, hypertension, hyperglycemia, and smoking. Recently, some viral infections have also been reported to contribute to the disease's development (18). Atherosclerosis may provide suitable conditions for SARS-CoV-2 viral propagation, and conversely, COVID-19 can influence atherosclerosis development by inducing plasmatic cytokines and inflammatory pathways. A meta-analysis revealed a higher incidence of hypertension and cardiovascular disease in severe cases among 53,000 COVID-19 patients (19). In this study, parameters related to fat metabolism such as triglycerides, LDL, and HDL; inflammatory pathways such as LDH; the renin-angiotensin system including ACE and ACE2; and the susceptibility of COVID-19 patients to atherosclerosis were examined as described previously.
Lipid metabolism could be affected in COVID-19 patients (20). Although only cholesterol levels among the lipid profiles in our study significantly differed between severe and non-severe cases, decreased HDL levels and increased total cholesterol, LDL, and triglyceride levels were observed with disease severity. Previous studies have reported reductions in total cholesterol and HDL in COVID-19 patients, but triglyceride levels and the cholesterol to HDL ratio were higher in those patients (20). A study by Wang et al. (21) reported the effect of higher tissue cholesterol content on COVID-19 severity due to vascular inflammation associated with tissue macrophages overloaded with cholesterol.
Low-density lipoprotein is the main lipoprotein accumulating in the sub-endothelial space and causes arterial wall inflammation after oxidation. TLR4/NFkB and JAK/STAT are inflammatory pathways involved in atherosclerosis, leading to induced inflammation, cytokine expression, and activation of the innate and adaptive immune systems (22, 23). High cholesterol levels trigger a pro-inflammatory state and increase patients' susceptibility to both COVID-19 and atherosclerosis.
As mentioned, the total cholesterol to HDL ratio is a good marker for the risk of cardiovascular diseases and atherosclerosis. The study results showed no significant difference in the chol/HDL ratio among patients with severe and non-severe COVID-19, but this difference was highly significant between the patients and the control group. The mean chol/HDL ratio was about 4 in both patient groups and 2.5 in healthy cases. Since ratio values above 3.5 are considered high risk for cardiovascular diseases (12), it can be concluded that COVID-19 increases the vulnerability to cardiovascular diseases and atherosclerosis.
TNF-α is an inducer for HDL and cholesterol levels. A previous study has shown that blocking TNF-α leads to an increase in total cholesterol and HDL levels (24). High-density lipoprotein has also been reported to decrease TNF-α levels by downregulating TLR-2, which is responsible for TNF-α production (25). IL-1 can induce IL-6, TNF-α, IL-8, and chemokines. CD14++ and CD16+, which are atherosclerosis-dependent monocytes, can also induce IL-1, TNF-α, and IL-6 (3). Increased levels of cytokines and switched immune cells are observed as inflammatory agents in both atherosclerosis and severe COVID-19 patients (26, 27). The current study showed an increase in total cholesterol levels in severe cases, but the HDL level was found to be decreased in those patients. Thus, TNF-α and IL-1 could be proper candidates for further investigations to find out the mechanism behind the changes in the lipid profile of the patients.
The importance of IL-1, IL-6, and TNF-α also makes them appropriate targets for therapies of inflammatory states in coronary heart disease and COVID-19 (3). Some commercial monoclonal antibodies have already been developed, including Canakinumab (IL-1b mAb) (28), Anakinra (IL-1R antagonist) (29), tocilizumab (IL-6R mAb) (30), and some anti-TNF antibodies which increase HDL levels for rheumatoid arthritis treatment including Etanercept, infliximab, and adalimumab (31-34). Low-dose colchicine treatment, which disrupts inflammasome activation, has also been proposed for controlling cardiovascular problems in patients with chronic coronary disease (35).
During COVID-19, the pro-inflammatory cytokines lead to oxidative stress due to the generation of free oxygen and halogen-containing radicals by endothelial and blood cells, which could develop atherosclerosis. These could lead to LDL oxidation, making it a neoantigen and leading to autoimmune processes in plaques (36).
Numerous studies at the pandemic's beginning reported cardiac injury in one-third of COVID-19 patients with increased levels of cardiac biomarkers such as troponin (37). SARS-CoV-2 infection induces many cytokines that can trigger a cytokine storm and atherosclerosis (2, 3). COVID-19 patients also show ST-segment elevation during electrocardiography, myocardial infarction, and acute myocarditis with very high levels of troponin (38, 39). Consistent with these results, our study showed a significant increase in troponin levels with disease severity, which implies the effect of COVID-19 infection on cardiac complications, including atherosclerosis.
CD147 is a glycoprotein from the immunoglobulin superfamily and acts as a SARS-CoV-2 receptor beside conventional ACE2. In our study, CD147 was similarly increased with disease severity, and its level was significantly higher in severe cases compared to non-severe. CD147 can induce inflammatory factors through the NF-kB pathway, and it has been reported to be upregulated in various inflammatory diseases (40).
In accordance with the results of this study, further investigations clarified that patients with normal levels of CD147, ACE, troponin, LDH, LDL, triglycerides, and cholesterol were less likely to experience severe disease and had a lower chance of being admitted to the ICU. Since CD147, ACE, and cholesterol were the distinct parameters with significant differences between severe and non-severe cases and also showed higher risk for disease severity in non-normal levels, they can be considered candidate biomarkers of COVID-19 severity. Among the mentioned parameters, only ACE and CPKmb levels were statistically significant risk factors.
MAPK and JAK/STAT are other signaling pathways that CD147 employs in pathological processes (40). Coronary artery disease also presents overexpression of CD147 in platelets, monocytes, granulocytes, and soluble forms (41). Low-density lipoproteins, CRP, advanced glycation end products, and high glucose are factors that can upregulate CD147 in inflammatory cells (42-44). CD147 is overexpressed in plaques and accumulates around macrophages, SMCs, and MMP-9-positive cells (45, 46). CD147 induces angiogenesis, and experiments have reported that inhibiting it with siRNA diminishes angiogenesis (47). In this study, the increased amounts of CD147 in severe COVID-19 cases also represent the higher risk for atherosclerosis development.
ACE2 on the surface of epithelial cells is the substantial receptor for SARS-CoV-2 (18). Both ACE and ACE2 are included in the renin-angiotensin system. In the present study, both ACE and ACE2 levels increased with disease severity, but only ACE was significantly different in comparison to severe and non-severe cases. The difference between the ICU (severe) and non-severe groups was not significant for ACE2, whereas the significant difference was between severe and non-severe groups compared to control groups. ACE2 has been reported to be upregulated in severe COVID-19 patients, and its increase is highly correlated with cardiovascular diseases (48-50). These observed differences include both soluble and cell-attached forms of ACE2. The ACE2/ACE ratio was previously reported to have decreased in most chronic diseases (51).
Angiotensin-converting enzyme 2 mediates the degradation of Ang II to Ang 1 - 7 and Ang I to Ang 1 - 9. Ang 1 - 7 plays anti-inflammatory roles, while ACE modulates inflammation. Viral replication downregulates ACE2 during virus entry into the host cell. Ang II leads to vast adverse effects, including myocardial hypertrophy, intestinal fibrosis, endothelial injury, increased inflammation, hypertension, oxidative stress, and higher levels of coagulation. It also activates the immune system, including macrophages, by inducing IL-6, TNF-α, and other inflammatory cytokines (52, 53). Thus, increasing soluble and membrane-attached ACE2 plays protective roles against COVID-19 (54).
A cholesterol-rich region in the membrane of host cells is related to virus attachment to the ACE2 receptor. In a study of cardiovascular patients, NLRP3 inflammasome is activated by intracellular cholesterol in macrophages, aiding the progression of both the COVID-19 cytokine storm and atherosclerosis. The scavenger class B receptor type 1 (SR-B1) is a cholesterol receptor protein that binds to HDL and interacts with the S1 subunit of the spike protein, facilitating better viral infection spread (55). Thus, cholesterol, HDL, and ACE2 work together in disease progression, cytokine storm, and the potential development of coronary artery disease. In this study, ACE2 was found to be correlated with both cholesterol and HDL.
Lactate dehydrogenase is a cytoplasmic enzyme present in various tissues, and its levels may increase in malignancies, lung disease, tissue injury, hypoxia, necrosis, myocardial ischemia, and hemolysis (56). Lactate dehydrogenase levels over 557 have been reported to be positively correlated with in-hospital mortality of cardiovascular patients (57). Both LDH and CRP have been reported to correlate with COVID-19 severity and have been proposed as predictors of respiratory failure in COVID-19 patients (58, 59), consistent with the results of this study. Lactate dehydrogenase also increased with disease severity in this study, which can be a triggering factor for developing atherosclerosis.
Our study shows that CPK and CPKmb levels were highest in non-severe patients in the ward, followed by ICU patients, and lowest in healthy cases. One study reported that CPK and CPKmb levels increased due to atherosclerosis and endothelial dysfunction (60). On the other hand, resveratrol, an atherosclerosis drug, inhibits the PI3K/AKT/mTOR pathway, leading to decreased CPK levels (60). Therefore, higher CPK and CPKmb levels in the patient (severe and non-severe) groups could represent a hypothetical maximum level that leads to the worst disease condition.
The results showed that CD147 correlated with troponin. CD147 and ACE2 contribute to virus infection by binding to the spike protein, and a strong correlation was found between them in this study. Thus, a hypothetical ratio of ACE2/CD147 was assessed among the studied groups. This ratio was significantly correlated with troponin. Since troponin is a cardiac biomarker and due to the intrinsic roles of ACE2 and CD147 in COVID-19 disease, the correlation of troponin with the ACE2/CD147 ratio can effectively link COVID-19 with atherosclerosis.
5.1. Conclusions
The results also showed that normal levels of CD147, ACE, Troponin, LDH, LDL, Triglyceride, and Cholesterol are associated with lower COVID-19 severity. Among these candidate biomarkers for COVID-19 severity, CD147, ACE, Troponin, and Cholesterol levels are the most promising due to their significant difference between severe and non-severe cases.
According to the present study on the relationship between atherosclerosis and COVID-19, and based on the results of recent studies, ACE and CD147 can be considered vital serum biomarkers in diagnosing atherosclerosis susceptibility in these patients. They may also serve as useful tools for monitoring and preventing the progression of atherosclerosis. This study suggests that CD147 and ACE levels could be potential biomarkers for assessing susceptibility to atherosclerosis among COVID-19 patients. Further investigations are recommended to confirm these findings. Our work should address limitations such as the small sample size and the examination of other inflammatory factors.