1. Background
Urinary tract infection (UTI) is the most common serious bacterial infection in febrile infants and young children, second only to otitis media, and more common than bacterial meningitis, pneumonia, and occult bacteremia. The UTI accounts for 10% of all febrile children, 13.6% of febrile infants, and 7% of febrile newborns. About 8% of girls and 2% of boys experience at least one episode of UTI during the first seven years of life. Early diagnosis and management of acute pyelonephritis (APN) are important to prevent its long-term complications, such as renal scars, proteinuria, hypertension, and chronic renal failure. However, more severe forms of acute febrile UTI may cause dehydration and subsequent prerenal azotemia (1, 2).
Vesicoureteral reflux (VUR) is one of the most common congenital abnormalities, affecting about 1% of healthy children. Meanwhile, 25% - 40% of children with acute febrile UTI are found to have VUR (2-5). Different predictive variables such as prenatal hydronephrosis, screening of a first-degree relative with hereditary VUR, UTI during the first year of life, recurrent UTI, severity of fever, non- Escherichia coli infections, level of C-reactive protein (CRP), and abnormal imaging studies have been suggested for the investigation of VUR, especially of high grades (6-11).
2. Objectives
This study was performed for the further evaluation of demographic, laboratory, and sonographic predictive parameters of VUR in a group of children with acute febrile UTI, as a complement to previous studies and to suggest performing selective cystography.
3. Methods
A retrospective study was conducted on the medical records of 424 children with acute febrile UTI who were admitted to a tertiary medical center and evaluated for VUR. Inclusion criteria consisted of children younger than 12 years old with the first symptomatic acute febrile UTI, with no other concomitant infectious or inflammatory disorders. Patients with chronic kidney disease, genetic syndromes, previous antibiotic treatment, other urologic abnormalities, and secondary VUR were excluded from the study.
Demographic and clinical characteristics of all patients were obtained from the medical records. Laboratory exams such as acute phase reactants [white blood cell count (WBC), erythrocyte sedimentation rate (ESR), CRP], serum creatinine (Cr), and urine culture were obtained at the time of admission and prior to antibiotic treatment. Ultrasonography was performed at the time of admission or during the next day.
Acute pyelonephritis was defined as a positive urine culture (any growth in suprapubic aspiration, > 105 CFU/mL of a single pathogen in urine bag collection, or > 104 CFU/mL in urethral catheterization) associated with temperature > 38.5°C, and increased WBC, ESR, or CRP values. Renal ultrasound was performed by a single expert pediatric radiologist using a Philips Affinity 50 ultrasound machine with linear and convex transducers. Sonographic findings of APN included increased kidney length, heterogeneity of renal parenchymal echogenicity, and increased urothelial thickness of the pyelocalyceal system.
Cystography was performed in patients with delayed response to antibiotic therapy within the first 48 - 72 hours of treatment, severely ill children, increased serum Cr, urosepsis, growth of atypical organisms, history of VUR in siblings or parents, and abnormal renal ultrasound findings such as renal scar, urinary tract dilatation, ectopic kidney, small kidney, and other kidney malformations. Cystography was done after obtaining the post-treatment negative urine culture. Grading of VUR was done according to the International Reflux Study Group as mild (grades 1 and 2), moderate (grade 3), and severe (grades 4 and 5). In cases of bilateral VUR, the more severe side was graded.
Statistical analysis was performed using SPSS version 27 for Windows (SPSS Inc., Chicago, IL, USA) and MedCalc 15.4 (MedCalc Software, Belgium). Data were shown as frequency and percentage for categorical variables, and mean ± SD for continuous variables. Categorical variables were compared between the two groups using chi-square and Fisher exact tests. Meanwhile, continuous variables were compared by the Mann-Whitney U test. Odds ratios were obtained by stepwise logistic regression analysis. Receiver operating characteristic (ROC) curves and area under the curve (AUC) were used to determine the proper cutoff of variables with the highest sensitivity, specificity, and accuracy. A P-value less than 0.05 was considered significant.
4. Results
A total of 424 children were included in this study. The mean age at the time of admission was 25.55 ± 32.09 months. Among them, 120 (28.3%) patients were male, and 304 (71.7%) were female. Escherichia coli was the most common causative organism of UTI in all patients, followed by Klebsiella and Pseudomonas.
All patients were divided into two groups: Those with VUR (n = 204) and those without VUR (n = 220). The majority of patients had bilateral VUR (41.17%), followed by left-sided VUR (33.33%) and right-sided VUR (25.5%). The VUR was mild in 25.48% of patients (3.92% grade 1 and 1.5% grade 2), followed by moderate VUR (grade 3) in 35.29%, and severe VUR in 39.23% of patients (23.03% grade 4 and 16.2% grade 5). The mean age at the time of the study showed no significant difference between the two groups (P = 0.061). Females outnumbered males in both groups; however, VUR was significantly more common in male patients (P = 0.001).
Serum Cr levels were significantly higher in children with VUR (P < 0.001). Normal ultrasound findings were more common in patients without VUR, whereas abnormal ultrasound findings were significantly associated with children with VUR (P < 0.001). Escherichia coli was the most common organism in both groups of patients; however, the growth of non-E.coli organisms was significantly more common in UTI cases complicated by VUR (P = 0.009). The demographic and clinical characteristics of the patients are summarized in Tables 1 and 2.
| Variables | With VUR (n = 204) | Without VUR (n = 220) | P-Value b |
|---|---|---|---|
| Mean age at diagnosis (mon) | 23.12 ± 31.74 | 27.80 ± 32.33 | 0.061 |
| Gender (M/F) | 73 (35.8)/131 (64.2) | 47 (21.4)/173 (78.6) | 0.001 |
| WBC (/mm3) | 15273.53 ± 6947.46 | 18033.63 ± 19312.73 | 0.103 |
| ESR (mm/h) | 59.96 ± 32.56 | 59.51 ± 29. 10 | 0.801 |
| CRP (mg/dL) | 71.77 ± 43.03 | 71.90 ± 41.14 | 0.991 |
| Cr (mg/dL) | 0.72 ± 0.52 | 0.56 ± 0.15 | < 0.001 |
| Abnormal ultrasound | 127 (62.3) | 84 (38.2) | < 0.001 |
Abbreviations: VUR, vesicoureteral reflux; M, males; F, females; Cr, creatinine; WBC, white blood cell count; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
a Values are expressed as mean ± SD or No. (%).
b P ≤ 0.001 was considered statistically significant.
| Urine Culture | With VUR | Without VUR | P-Value |
|---|---|---|---|
| Escherichia coli | 137 (67.15) | 180 (81.81) | 0.009 b |
| Klebsiella | 29 (14.21) | 23 (10.45) | |
| Pseudomonas | 14 (6.86) | 6 (2.72) | |
| Enterobacter | 12 (5.89) | 5 (2.3) | |
| Others | 12 (5.89) | 6 (2.72) |
Abbreviation: VUR, vesicoureteral reflux.
a Values are expressed as No. (%).
b P ≤ 0.01 was considered statistically significant.
Univariate logistic regression analysis showed that gender, serum Cr, and abnormal ultrasound had a significant correlation with VUR. However, in multivariate analysis, only serum Cr [OR: 14.830, 95% CI (3.951 - 55.661), P < 0.001] and abnormal renal ultrasound [OR: 2.063, 95% CI (1.323 - 3.216), P = 0.001] had an independent correlation with VUR (Table 3).
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value a | OR (95% CI) | P-Value a | |
| Age | 0.995 (0.989 - 1.001) | 0.136 | 0.988 (0.981-0.996) | 0.06 |
| Gender | 2.051 (1.333 - 3.157) | 0.001 | 1.211 (0.739-1.984) | 0.447 |
| Cr | 13.523 (4.342 - 42.112) | < 0.001 | 14.830 (3.951-55.661) | < 0.001 |
| Ultrasound | 2.670 (1.803 - 3.954) | < 0.001 | 2.063 (1.323-3.216) | 0.001 |
| Urine culture | ||||
| Klebsiella | Ref | Ref | Ref | Ref |
| Escherichia coli | 0.604 (0.334 - 1.090) | 0.754 | 0.903 (0.478 - 1.706) | 0.754 |
| Pseudomonas | 1.850 (0.615 - 5.570) | 0.118 | 2.534 (0.790 - 8.127) | 0.118 |
| Enterobacter | 1.903 (0.586 - 6.183) | 0.223 | 2.816 (0.792 - 10.017) | 0.223 |
Abbreviation: VUR, vesicoureteral reflux; Cr, creatinine
a P ≤ 0.001 was considered statistically significant.
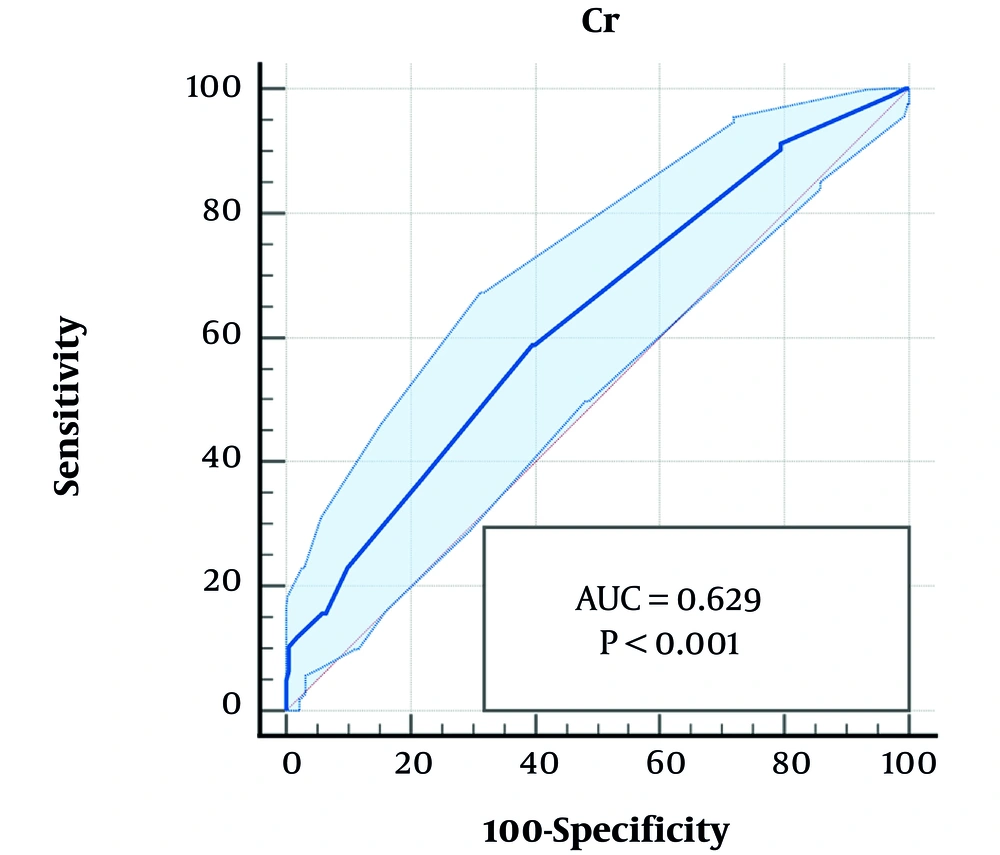
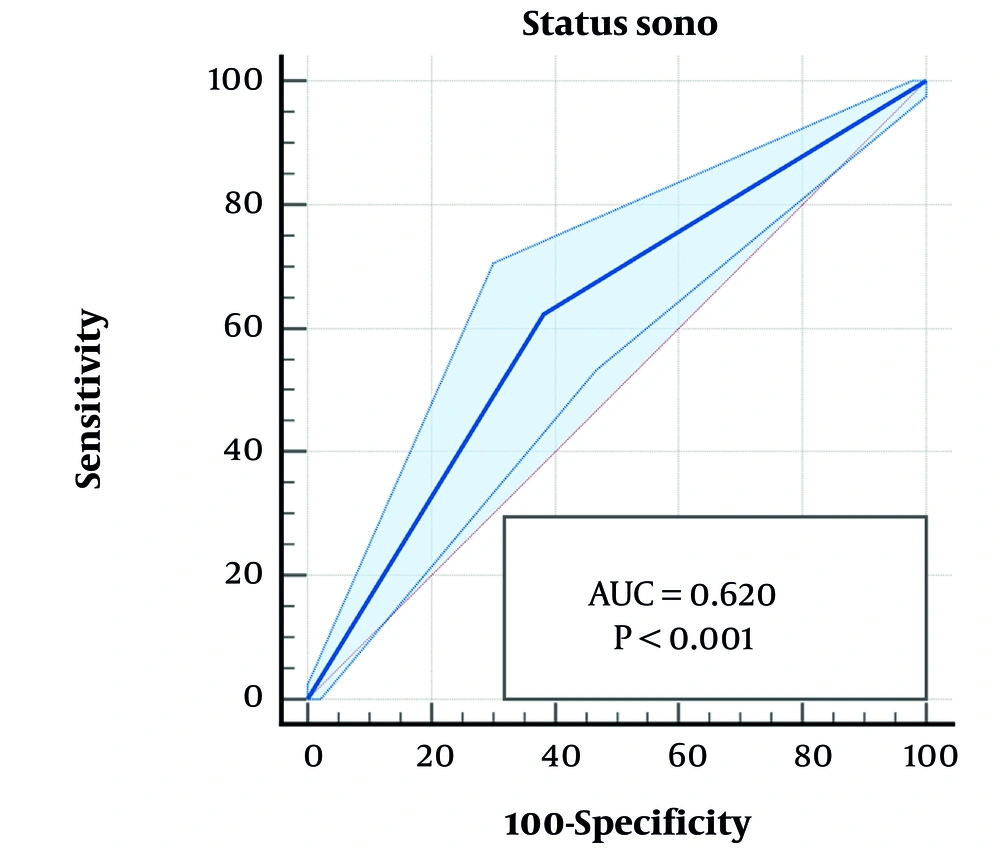
The area under the ROC curve analysis showed that serum Cr (P < 0.001, 95% CI: 0.581 - 0.675) and abnormal renal ultrasound (P < 0.001, 95% CI: 0.572 - 0.667) were accurate predictive parameters of VUR, though with moderate sensitivity and specificity, respectively (Table 4, Figures 1 and 2).
| Variables | Cutoff | Sensitivity | Specificity | AUC | 95% CI | Standard Error | P-Value a |
|---|---|---|---|---|---|---|---|
| Cr | 0.56 | 58.82 | 60.45 | 0.629 | 0.581 - 0.675 | 0.026 | < 0.001 |
| Ultrasound | 0 | 62.25 | 61.82 | 0.620 | 0.572 - 0.667 | 0.024 | < 0.001 |
Abbreviations: Cr, creatinine
a P ≤ 0.001 was considered statistically significant.
5. Discussion
Vesicoureteral reflux is one of the most important risk factors for UTI in the pediatric age group. It has been reported in 30% - 40% of children with UTI, suggesting that more than 60% of cystographic examinations might be unnecessary (12). The purpose of this study was to identify the value of demographic, laboratory, and sonographic findings that are suggestive of VUR in children with acute febrile UTI. Therefore, cystographic examination was performed in all of our patients, and they were compared in two groups: With and without VUR.
We found that male gender was more common in children with VUR. Similarly, some previous studies showed a higher incidence of male gender among children with VUR (13), especially in those with higher grades of VUR (5). However, VUR was more common in females in the study by Bahat et al. (14). Serum Cr was significantly higher in children with VUR in our study, indicating a higher incidence of concurrent or previous renal parenchymal damage in these patients. This finding was in accordance with some other studies, especially those with higher grades of VUR (15, 16).
Escherichia coli was the most common organism of UTI in all of our patients, in accordance with the majority of previous reports. However, non-E.coli organisms such as Klebsiella, Pseudomonas, and Enterobacteriaceae were significantly predominant in our patients with VUR. Abnormal pathogenic organisms were more common in children with VUR in other reports, especially in high-grade VUR, and those with even normal renal ultrasound (14, 15, 17-21).
The VUR was more common in younger children with UTI in our study, although not significant. This was also evident in some other studies with conflicting results. The VUR was more common in infants and young children in some reports (5, 13, 14, 17). However, in other studies, VUR was suggested to be more prevalent in the diagnostic age of UTI > 6 months or late-onset UTI (18, 22).
Evaluation of acute phase reactants such as WBC, ESR, and CRP showed no significant difference between the two groups of our patients. However, a significant increase in these inflammatory markers was reported in some studies, especially in those with high-grade VUR and higher amounts of these parameters (4, 13, 22-24).
Escherichia coli was the most prevalent organism in both groups of patients with and without VUR. However, the growth of non-E.coli enteric organisms was more common in those with VUR. This finding has been reported in the majority of previous studies (14, 15, 17-21), indicating an increased risk of unusual pathogenic organisms in urologic abnormalities. Serum Cr levels greater than 0.56 mg/dL were a predictive marker of VUR in our study. Serum Cr was significantly higher in patients with VUR compared to those without VUR, suggesting higher renal parenchymal inflammation during the acute phase of febrile UTI with VUR or pre-existing renal parenchymal damage in those with VUR. Increased serum Cr was also considered a predictor of VUR, especially of higher grades, in some similar studies (15, 16). An increased incidence of abnormal renal bladder ultrasound was another significant finding in children with VUR in our study, highlighting the importance of radiologic examination in children with acute febrile UTI for early diagnosis and appropriate management of VUR to prevent further renal damage and the development of chronic kidney disease. Abnormal sonographic findings have been reported as a marker of high-grade VUR in previous studies (5, 14, 15, 17, 23-26). Both increased serum Cr and sonographic abnormalities had a significant correlation with the presence of VUR in our multivariate analysis. According to the ROC curve analysis, these two variables were accurate markers of VUR with moderate sensitivity and specificity.
5.1. Conclusions
In the present study, increased serum Cr and abnormal renal bladder ultrasound were more common findings in children with acute febrile UTI and VUR compared to those without VUR. These two variables were accurate markers of VUR with moderate sensitivity and specificity. However, as data were collected from a single center, they cannot be completely generalized, and multicenter studies are suggested to increase the reliability of these findings.