1. Introduction
Autoimmune hepatitis (AIH) is a heterogeneous condition characterized by the loss of immune tolerance to liver-specific antigens (1). The loss of immune tolerance against the patient's liver antigens is considered the main pathogenic mechanism. Its classification often relies on underlying etiologies and disease severity. Genetic predisposition, when combined with environmental triggers such as viruses, can initiate or exacerbate the pathogenic process (2, 3). During the COVID-19 pandemic, SARS-CoV-2 has emerged as a significant environmental factor.
The cycle threshold (Ct) value in PCR testing serves as a critical indicator of viral load; lower Ct values (≤ 20) imply a high viral burden and increased infectivity, while Ct values above 30 suggest reduced viral presence typical of later infection stages or recovery. COVID-19 typically unfolds in distinct phases: Early symptoms arise within the first five days; respiratory distress may develop between days 5 and 10; peak immune activation commonly appears between days 10 and 14; and recovery begins thereafter, although residual symptoms may persist beyond this period. In some cases, jaundice emerges two to four weeks after infection. This presentation could result from systemic inflammation, direct viral effects on hepatic cells, or drug-induced liver injury. Individuals with pre-existing liver conditions or significant inflammatory responses face heightened risk. Understanding the molecular and pathophysiological mechanisms underlying COVID-19-associated liver dysfunction is crucial for developing targeted therapeutic interventions (4).
Intriguingly, beyond the well-characterized angiotensin-converting enzyme 2 (ACE2)-mediated entry, SARS-CoV-2 may infect hepatic cells using alternative receptors such as neuropilin 1 (NRP1) or CD147. Additionally, proteases like TMPRSS2, furin, and cathepsins facilitate spike protein activation, potentially contributing to cell injury via endoplasmic reticulum stress, mitochondrial dysfunction, and impaired autophagy that culminate in disrupted lipid metabolism and reactive oxygen species (ROS) accumulation (5).
A further complexity in the current landscape involves the immune response following COVID-19 vaccination. Anecdotal evidence suggests that immune activation after vaccination, particularly with heterologous regimens (e.g., primary vaccination with AZD1222 followed by an mRNA booster), may in rare instances be associated with AIH (6).
Complications related to immune activation following AstraZeneca’s COVID-19 vaccine can emerge within 5 to 30 days after vaccination. COVID-19 vaccination-induced AIH may be linked to mechanisms such as molecular mimicry, adjuvants, epitope spreading, bystander activation, X chromosome effects, and SARS-CoV-2’s hepatotropism (7, 8).
Proposed mechanisms include molecular mimicry, adjuvant effects, epitope spreading, bystander activation, and possible X chromosome influences. Observations indicate that after contracting the COVID-19 virus, reports of AIH flares have increased, leading to research on the relationship between COVID-19 and chronic hepatitis: Is it an infectious agent or a cause for deterioration?
2. Case Presentation
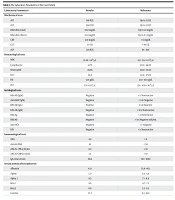
A 48-year-old woman with no history of underlying diseases visited the doctor on August 15, 1401, in Tabriz with symptoms of anorexia, runny nose, and mild cough. After conducting the PCR test for COVID-19, the test result was positive. After 5 days, coryza symptoms were resolved, but her anorexia continued, and intravenous vitamins, intravenous serum, and pantoprazole were prescribed. The patient did not receive any antiviral medication. Ten days after the onset of the disease, the urine color changed from yellow to brown, which was accompanied by the yellowing of the sclera and skin. In clinical examinations, organomegaly and lymph nodes were not palpable in the abdomen. Direct and indirect bilirubin tests were elevated. Ultrasound results did not show biliary obstruction, and the echogenicity of the liver was normal. In the tests, viral hepatitis was negative. Due to the increase in liver enzymes, a liver biopsy was performed, and chronic hepatitis was reported. Electrophoresis of serum proteins indicated liver necrosis. The liver enzymes rose post-recovery from the disease. The autoimmune tests performed showed an increase in the IgG marker. The past medical history reveals that the patient was diagnosed with COVID-19 on May 12, 2019, which was accompanied by lung inflammation in the CT scan. The patient has a history of receiving three doses of the AstraZeneca vaccine. Table 1 shows the laboratory parameters of the case patient in this study.
| Laboratory Parameters | Results | Reference |
|---|---|---|
| Biochemical tests | ||
| AST | 818 IU/L | Up to 33 U/L |
| ALT | 500 IU/L | Up to 33 U/L |
| Bilirubin (total) | 8.62 mg/dL | Up to 1.4 mg/dL |
| Bilirubin (direct) | 8.03 mg/dL | Up to 0.3 mg/dL |
| CRP | 12.9 mg/dL | < 6 mg/dL |
| GGT | 177 U/L | < 40 U/L |
| ALP | 239 IU/L | 64 - 306 |
| Hematological tests | ||
| WBC | 12.24 × 103/ µL | 4.0 - 12.4 × 103/ µL |
| Lymphocyte | 51.7% | 20.0 - 48.0% |
| Neutrophil | 39.8% | 40.0 - 74.0% |
| HCT | 34.8 | 34.0 - 47.0% |
| Hb | 12.4 g/dL | 12.0 - 16.0 g/dL |
| PLT | 374 × 103/ µL | 130 – 450 × 103/ µL |
| Serological tests | ||
| HBc-Ab (IgM) | Negative | < 1: Nonreactive |
| Anti-HAV (IgM) | Negative | < 0.9: Negative |
| EBV-Ab (IgG) | Positive | ≥ 1.0: Reactive |
| EBV-Ab (IgM) | Negative | < 0.5: Nonreactive |
| HBS-Ag | Negative | < 1: Nonreactive |
| HBS-Ab | Negative | < 10: Negative mIU/mL |
| Anti-HCV | Negative | < 1: Negative |
| HIV | Negative | < 1: Nonreactive |
| Immunological tests | ||
| ANA | 4.6 | < 8 |
| Anti-ds DNA | 19 | < 50 |
| ANCA-C (PR-3) IU/mL | 8.9 | < 10 |
| ANCA-P (MPO) IU/mL | 7.4 | < 10 |
| IgG total serum | 3014 | 700 - 1600 |
| Serum protein electrophoresis | ||
| Albumin | 43.1 | 55.8 - 66.1 |
| Alpha 1 | 5.0 | 2.9 - 4.9 |
| Alpha 2 | 9.5 | 7.1 - 11.8 |
| Beta 1 | 8.6 | 4.7 - 7.2 |
| Beta 2 | 6.6 | 3.2 - 6.5 |
| Gamma | 27.2 | 11.1 - 18.8 |
The Laboratory Parameters of the Case Patient
3. Discussion
This case highlights a temporal association between SARS-CoV-2 infection and the onset of hepatitis, potentially indicative of AIH, given the elevated liver enzymes and raised IgG levels following infection. Since this disease followed the infection of COVID-19, the question arises whether COVID-19 can ignite this disease. It is reported that nearly 68% of adults during the COVID-19 epidemic demonstrated evidence of AIH, with higher rates observed among those with respiratory complications (9).
Furthermore, autopsy studies have revealed histopathological signs of autoimmune-mediated liver damage in patients who succumbed to COVID-19 (10). The ACE2 receptor expression in bile ducts has been explored in various studies, including work by Zheng et al. (8).
The ACE2 receptor, which facilitates SARS-CoV-2 entry into host cells, is expressed in cholangiocytes, suggesting a potential mechanism for direct viral infection of bile duct cells. This could contribute to liver dysfunction observed in COVID-19 patients, as bile duct injury may lead to cholestasis and impaired liver function. Regarding cytokine storm-mediated liver damage, interleukin-6 (IL-6) plays a crucial role in AIH flares. Excessive IL-6 signaling can drive immune dysregulation, exacerbating liver inflammation and fibrosis. In COVID-19, elevated IL-6 levels have been linked to severe systemic inflammation, potentially worsening pre-existing autoimmune liver conditions. Targeting IL-6 pathways has been considered a therapeutic strategy to mitigate liver injury in severe cases (11).
These factors can lead to the development of autoantibodies and autoimmune diseases. COVID-19 can activate or hyper-stimulate the immune system, or due to the similarity of foreign peptides with the peptides of the human body (molecular mimicry), it can lead to an increase in autoimmune liver diseases (12).
On the other hand, T lymphocytes (CD3+ and CD8+) can play an important role in triggering the process of autoimmune diseases by damaging the tissues (9).
Despite reports of AIH occurring post-COVID-19, the Efe et al.’s study found no significant increase in AIH cases following SARS-CoV-2 infection. Discrepancies across studies may be explained by variations in host genetics, which influence immune response and disease susceptibility. This inconsistency underscores the necessity for further research into COVID-19’s role in autoimmune liver disease (12).
Given the possibility of molecular mimicry between viral antigens and liver-specific targets, testing for anti-SARS-CoV-2 antibodies that cross-react with liver antigens such as LKM-1 could clarify the potential autoimmune mechanisms underlying post-COVID liver dysfunction (13).
Another immunological concern is Epstein-Barr virus (EBV) reactivation in COVID-19 patients. Reactivation of EBV has been associated with AIH, as it can contribute to autoimmune diseases through molecular mimicry, B cell alterations, and immune system dysregulation. Typically, anti-EBV IgM levels increase in the early stages of infection, signaling acute EBV hepatitis, while anti-EBV IgG rises later, reflecting a prior infection or sustained immune response. However, since these markers were absent in this case, it is unlikely that EBV played a role in the hepatitis observed (14).
Recent literature also explores immune-mediated hepatitis linked to AstraZeneca’s vaccine, potentially involving vaccine-induced immune thrombotic thrombocytopenia (VITT)-like mechanisms. Understanding how vaccination may contribute to autoimmune liver pathology is critical for post-vaccination screening. The VITT is a rare but serious adverse effect associated with adenoviral vector-based vaccines, including AstraZeneca's ChAdOx1 nCoV-19. Studies highlight the role of anti-platelet factor 4 (PF4) antibodies in triggering thrombosis and thrombocytopenia, resembling heparin-induced thrombocytopenia (HIT) (15).
3.1. Conclusions
Screening for liver dysfunction in post-COVID-19 patients is crucial, as emerging research indicates that COVID-19 can cause long-term liver damage, even months after recovery. Studies have shown that patients with persistent symptoms may exhibit elevated liver stiffness, which could signal fibrosis or chronic inflammation. Additionally, research suggests that post-COVID hepatic manifestations may include steatosis, necroinflammation, and secondary biliary cholangitis, particularly in individuals with pre-existing liver conditions (16).
3.2. Limitations
We acknowledge that while molecular mimicry is a plausible mechanism in the potential link between SARS-CoV-2 and AIH, epitope mapping has not provided definitive support for this hypothesis. Given this limitation, we recognize the importance of exploring cross-reactivity testing to assess whether antibodies against the SARS-CoV-2 spike protein might also target liver antigens.