1. Background
Providencia species are gram-negative bacteria that are part of the microbiota of the human gastrointestinal tract. These opportunistic pathogens are rarely responsible for nosocomial infections (1). Providencia stuartii and P. rettgeriiare the most pathogenic Providencia species, primarily causing urinary tract infections (UTIs), especially in patients with urinary catheters. Additionally, they can lead to pneumonia, meningitis, endocarditis, septicemia, and surgical site infections (SSI) (2).
The genus Providencia possesses numerous virulence characteristics, including biofilm formation, attachment to host cells via fimbriae, and lysis of red blood cells by haemolysin, thereby rendering them formidable pathogens (3). Furthermore, they carry antibiotic-resistance genes on either their chromosome or plasmids, making them resistant to antimicrobial agents such as colistin, tigecycline, and carbapenems. Hence, these multiple-drug-resistant (MDR) pathogens with the aforementioned virulence characteristics can spread in hospital settings and cause fatal outbreaks (4). Despite its rarity, infections with Providencia spp. can result in a high mortality rate, particularly in those with primary bacteremia (5). Moreover, treating Providencia infections is often challenging for clinicians due to the high drug-resistance rate (6). Thus, clinicians should expeditiously explore and manage these outbreaks to diminish morbidity and mortality.
2. Objectives
In this case series, we present an outbreak caused by PDR Providencia species in an intensive care unit (ICU), which was managed through timely diagnosis, therapeutic strategies, and effective preventive measures. This information may be instructive for healthcare workers dealing with similar outbreaks.
3. Methods
An outbreak of Providencia species occurred in the ICU at Imam Hossein Hospital, Tehran, Iran, between 20 June and 28 July 2023. The clinical and paraclinical features of the patients are summarized in Table 1. The majority of the 14 patients diagnosed with PDR Providencia spp. infection experienced ventilator-associated pneumonia (VAP). However, two others had SSI and peritoneal abscesses. Initially, Providencia spp. were isolated from three patients, which was reported to the infection prevention and control (IPC) committee. Upon visiting the infected patients, the IPC committee recommended that contact precautions be taken. Consequently, these patients were isolated from other patients in separate rooms. Subsequently, the IPC committee convened a meeting with the hospital administrators, and the following decisions were made:
- Isolation of patients infected with PDR Providencia in a separate closed ward.
- Sampling from other patients (urine culture (U/C), blood culture (B/C), and tracheal specimens), the hands of healthcare workers, and the environment in the ICU.
- Disinfection of the ICU with a 1% chlorine solution.
- Employment of specially trained healthcare workers for these patients.
- Observance of contact precautions by healthcare workers, including hand hygiene, gloves, scrubs, and shoe covers.
- Daily observation by the IPC committee of this separate closed ward.
- Sampling from patients and the environment after antibiotic therapy (meropenem, piperacillin/tazobactam, and amikacin, as mentioned below) and environmental disinfection.
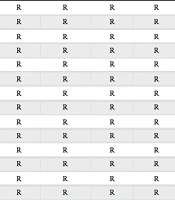
Samples were inoculated into peptone water at 37ºC for 24 hours. They were then subcultured on blood Agar, chocolate Agar, and MacConkey Agar, and incubated at 37ºC for 24 hours. The suspected Providencia spp. colonies with a pale appearance on MacConkey Agar were picked, and differential gallery mediums were applied: Triple Sugar Iron Agar (T.S.I.), Sulfide Indo Motility Medium (S.I.M.), Simmon's Citrate Agar (S.C.), Urea Broth (U), and Methyl Red Voges Proskauer Broth (MR.VP). All mediums were produced by Condalab Company, Madrid, Spain (batch number 312211). After sampling from all patients admitted to the ICU, 11 more patients infected with PDR Providencia spp. were identified. Furthermore, susceptibility to antimicrobial agents was determined by the disk diffusion protocol using Mueller-Hinton (MH) agar according to clinical & laboratory standards institute (CLSI) standards (7). Antibiotic disks and the medium were produced by Rosco Diagnostica A/S DK-2630-Albertslund, Denmark. Table 2 illustrates the antibiotic resistance patterns of the isolates.
| Patient Number | Sex, Age | Underlying Diseases | Duration of ICU Hospitalization Before Detecting Providencia spp. (days) | Treatment with Broad-Spectrum Antibiotics | Lung Imaging | WBC (103/μL) a | ESR (mm/h) | CRP (mg/dL) | PCT (ng/mL) | Lactate (mmol/L) | Site of Isolation | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M, 77 | CVA | 22 | Meropenem, 16 days | Bilateral consolidation | 11600 | 120 | > 200 | 4.8 | 32 | Tracheal secretions | AKI + VAP | Expired |
| 2 | F, 68 | Colon cancer | 35 | Cefepime, 25 days | Left-side consolidation | 26800 | 120 | 168 | 2.69 | 18 | Tracheal secretions | ECF + VAP | Expired |
| 3 | F, 72 | DM, IHD | 39 | Meropenem + ampicillin/sulbactam, 12 days | Right-side consolidation | 18600 | 120 | 94 | 1.38 | 47 | Tracheal secretions | VAP | Survived |
| 4 | M, 39 | - | 15 | Meropenem + Amikacin, 15 days | Normal | 20900 | 120 | 105 | 0.38 | 8 | Wound secretions | SSI | Survived |
| 5 | F, 39 | Cervical cancer | 15 | Ceftriaxone + Metronidazole, 15 days | Normal | 18800 | 120 | 96 | 1.7 | 21 | Peritoneal abscess | Peritoneal abscess | Survived |
| 6 | M, 64 | Pulmonary emphysema | 16 | Ceftriaxone + Clindamycin, 13 days | Bilateral pleural effusion | 6500 | 10 | 14.1 | 0.01 | 43 | Pleural fluid | Distal radius Fracture + VAP | Survived |
| 7 | F, 50 | - | 41 | Ceftriaxone + Clindamycin, 40 days | Bilateral patchy infiltrations | 34300 | 120 | 85 | 0.5 | 15 | Tracheal secretions | Vertebral fracture + VAP | Expired |
| 8 | M, 48 | DM, HTN | 40 | Ceftriaxone + Clindamycin, 25 days | Bilateral pleural effusion + Bilateral consolidation | 14400 | 111 | 89 | 0.37 | 17 | Tracheal secretions | Hypoglycemia + VAP | Expired |
| 9 | M, 80 | CVA | 8 | Meropenem, 8 days | Mild haziness in both lungs | 15500 | 37 | 53 | 0.27 | 16 | Tracheal secretions | IVH + SAH + VAP | Survived |
| 10 | M, 30 | - | 8 | Meropenem, 8 days | Bilateral consolidation + Patchy infiltrations | 29300 | 120 | 75 | 5.2 | 23 | Blood culture | Double fracture of the leg + VAP | Expired |
| 11 | F, 86 | Alzheimer's disease | 10 | Meropenem + Colistin, 9 days | Patchy infiltrations | 21500 | 120 | 96 | 18.4 | 17 | Tracheal secretions | SDH + VAP | Expired |
| 12 | F, 66 | HTN | 18 | Meropenem, 12 days | Necrosan infiltrations | 32700 | 120 | 71 | 10.2 | 61 | Tracheal secretions | CVA + VAP | Expired |
| 13 | M, 19 | - | 20 | Piperacillin/tazobactam, 14 days | Right-side consolidation | 17300 | 120 | 90 | 8.09 | 17 | Tracheal secretions | EDH + VAP | Survived |
| 14 | - | 60 | Meropenem, 60 days | Normal | 33000 | 120 | 91 | 1.15 | 16 | Tracheal secretions | Diffuse axonal injury + VAP | Expired |
Abbreviations: AKI, acute kidney injury; CRP, C-reactive protein; CVA, cerebrovascular accident; DM, diabetes mellitus; ECF, entrocutaneous fistula; EDH, epidural hemorrhage; ESR, erythrocyte sedimentation rate; F, female; HTN, hypertension; IHD, ischemic heart disease; IVH, intranebtricular hemorrhage; M, male; PCT, procalcitonin; SAH, subarachnoid hemorrhage; SDH, subdural hemorrhage; SSI, surgical site infection; VAP, ventilator-associated pneumonia; WBC, white blood cell; ICU, intensive care unit.
a All patients with leukocytosis had polymorphonuclear (PMN) dominant pattern.
| Patient Number | Amikacin | Ampicillin/ Sulbactam | Cefepime | Cefotxime | Ceftazidime | Colistin | Gentamicin | Imipenem | Meropenem | Levofloxacin | Piperacillin/ Tazobactam | Tetracyclin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R | R | R | R | R | R | R | R | R | R | R | R |
| 2 | R | R | R | R | R | R | R | R | R | R | R | R |
| 3 | R | R | R | R | R | R | R | R | R | R | R | R |
| 4 | R | R | R | R | R | R | R | R | R | R | R | R |
| 5 | R | R | R | R | R | R | R | R | R | R | R | R |
| 6 | R | R | R | R | R | R | R | R | R | R | R | R |
| 7 | R | R | R | R | R | R | R | R | R | R | R | R |
| 8 | R | R | R | R | R | R | R | R | R | R | R | R |
| 9 | R | R | R | R | R | R | R | R | R | R | R | R |
| 10 | R | R | R | R | R | R | R | R | R | R | R | R |
| 11 | R | R | R | R | R | R | R | R | R | R | R | R |
| 12 | R | R | R | R | R | R | R | R | R | R | R | R |
| 13 | R | R | R | R | R | R | R | R | R | R | R | R |
| 14 | R | R | R | R | R | R | R | R | R | R | R | R |
Abbreviations: R, resistant; S, sensitive.
a Base on the antibiotic resistance patterns, all Providencia spp. isolated from the patients were pan-drug resistant.
The patients were treated with a combined regimen as follows: Meropenem (2 g every 8 hours intravenous infusion with slow pump over 3 hours), piperacillin/tazobactam (4.5 g every 6 hours intravenous infusion without slow pump), and amikacin (500 mg every 12 hours intravenously and/or nebulized for VAP). The drugs were adjusted based on glomerular filtration rate (GFR) as indicated.
4. Results
The first case was a 77-year-old man with a history of cerebrovascular accident (CVA), who complained of cough, dyspnea, and fever. His lung computed tomography (CT) scan displayed bilateral consolidation within the lung parenchyma. Additionally, laboratory results revealed an increased serum creatinine level (6.1 mg/dL). Thus, he was intubated and hospitalized in the ICU with the diagnosis of acute kidney injury in addition to pneumonia. Despite receiving intravenous meropenem for 16 days, the patient's symptoms did not improve, prompting us to investigate further into the disease’s etiology. The tracheal secretions of the patient were positive for Providencia species. His clinical manifestations deteriorated despite antibiotic treatment, and he expired 11 days later.
The second case was a 68-year-old woman with a history of colon cancer who suffered from an enterocutaneous fistula following a total colectomy. She was intubated and hospitalized in the ICU after a surgical operation and drain placement. Despite receiving intravenous cefepime for 25 days, she was not eligible to be weaned from the ventilator. Her lung CT scan displayed consolidation in the left lung, and her tracheal secretions were positive for Providencia species. She was treated with the diagnosis of VAP. However, her clinical manifestations deteriorated, and she expired 11 days later.
The third case was a 72-year-old woman with a history of diabetes mellitus (DM) and ischemic heart disease (IHD) who complained of drowsiness, cough, nausea, and coffee-ground vomiting. Her lung CT scan displayed consolidation in the right lung. She was intubated and hospitalized in the ICU due to decreased level of consciousness and respiratory distress. She was deteriorating despite receiving intravenous meropenem and ampicillin/sulbactam for 12 days to treat pneumonia. Her tracheal secretions were positive for Providencia species upon further investigations. After changing her antibiotic regimen, she improved dramatically and was discharged 11 days later.
The fourth case was a 39-year-old man without any underlying disease who was hospitalized after a passenger motor accident. He underwent surgical operations and vacuum therapy to treat a plateau fracture of his left tibia. Despite receiving intravenous meropenem and amikacin, purulent secretions emerged from the wound. The specimen obtained from the wound tested positive for Providencia species. He was treated with an appropriate antibiotic regimen and was discharged after improvement.
The fifth case was a 39-year-old woman with a history of cervical cancer who was diagnosed with a rectovaginal fistula. Consequently, a surgical operation was performed for fistula closure. Despite receiving intravenous ceftriaxone and metronidazole, a peritoneal abscess emerged after 17 days. The patient underwent surgery to drain the abscess and wash the peritoneal cavity. The specimen obtained from the abscess tested positive for Providencia species. She was treated with an appropriate antibiotic combination and discharged after improvement.
The sixth case was a 64-year-old man with a history of pulmonary emphysema, diagnosed with a distal radius fracture after a fall. Following a surgical procedure for percutaneous pinning, he was hospitalized in the ICU and required mechanical ventilation. A chest X-ray showed evidence of bilateral massive pleural effusion, which was drained by chest tube insertion. The pleural fluid obtained through drainage tested positive for Providencia species. After treatment with intravenous antibiotics and considerable improvement, he was discharged from the hospital.
The seventh case was a 50-year-old woman without any underlying disease who was hospitalized in the ICU due to paraplegia and vertebral fractures following a car accident. After neurosurgery, her lung CT scan revealed bilateral patchy infiltrations. The tracheal secretions of the patient tested positive for Providencia species. She expired after 13 days, despite being given antibiotics to treat VAP.
The eighth case was a 48-year-old man with a history of DM and hypertension (HTN) who was diagnosed with hypoglycemia resulting from excessive insulin injections in a suicide attempt. He was intubated and hospitalized in the ICU. Despite receiving intravenous ceftriaxone and clindamycin for 25 days, he could not be weaned from the ventilator. His lung CT scan displayed bilateral consolidation and pleural effusion, and his tracheal secretions were positive for Providencia species. He expired after 19 days, despite being given antibiotics to treat VAP.
The ninth case was an 80-year-old man with a history of CVA referred to our medical center complaining of a decreased level of consciousness. He was admitted to the ICU with the diagnosis of intraventricular hemorrhage (IVH) and subarachnoid hemorrhage (SAH). During hospitalization, his chest X-ray revealed mild haziness in both lungs, indicating lobar pneumonia. Because his tracheal secretions were positive for Providencia species, he received antibiotics to treat VAP. He was discharged approximately 15 days later, exhibiting complete improvement.
The tenth case was a 32-year-old man without any underlying disease who was hospitalized after a motor car accident. He underwent surgical operations to install external fixators for a double fracture of the leg. He developed respiratory distress a week after surgery. Consequently, he was intubated and admitted to the ICU. Despite receiving intravenous meropenem, he deteriorated, prompting further investigation. His lung CT scan displayed bilateral consolidation and patchy infiltrations. The blood culture of the patient was positive for Providencia species. Eight days after treatment for VAP, he expired as a result of sepsis and multiple organ failure.
The eleventh case was an 86-year-old woman with a history of Alzheimer's disease who was hospitalized due to loss of consciousness following a fall. She was intubated and admitted to the ICU with the diagnosis of subdural hemorrhage (SDH). Despite receiving intravenous meropenem and colistin, she developed VAP. Her lung CT scan displayed patchy infiltrations in both lungs. Additionally, her tracheal secretions were positive for Providencia species. She expired three weeks later secondary to sepsis and multiple organ failure.
The twelfth case was a 66-year-old woman with a history of HTN who complained of imbalance. She was intubated and admitted to the ICU with the diagnosis of CVA. She developed VAP about two weeks later. Her lung CT scan displayed bilateral necrotic infiltrations. Her tracheal secretions were also positive for Providencia species. She expired after 11 days, despite being given antibiotics to treat VAP.
The thirteenth case was a 19-year-old man without any underlying disease who was hospitalized due to loss of consciousness following a motor accident. He was diagnosed with epidural hemorrhage (EDH) and underwent a craniotomy. Despite receiving intravenous piperacillin/tazobactam, he developed VAP about three weeks later. His lung CT scan displayed consolidation in the left lung, and his tracheal secretions were positive for Providencia species. After treatment with intravenous antibiotics and considerable improvement, he was discharged from the hospital.
The last case was a 19-year-old man without any underlying disease who was hospitalized due to loss of consciousness following a car accident. He was intubated and admitted to the ICU with the diagnosis of diffuse axonal injury. Despite receiving intravenous meropenem, he subsequently developed VAP about 60 days later. His tracheal secretions were positive for Providencia species. He expired after 9 days as a result of sepsis and multiple organ failure.
All samples obtained from healthcare workers and the environment (ICU floor, ICU walls, tables, beds, and ventilators) before and after disinfection were negative for Providencia species. Furthermore, surviving patients were discharged with negative cultures.
5. Discussion
An outbreak caused by Providencia species occurred among patients hospitalized in the ICU, which was promptly diagnosed and managed through preventive and therapeutic measures recommended by the IPC committee.
The clinical manifestations in published articles about Providencia outbreaks in ICU-admitted patients were dominated by UTIs (1, 8-11). Most patients requiring intensive care have urinary catheters, which predispose them to UTIs. Furthermore, the urine of chronically catheterized patients may serve as a reservoir for uncommon pathogens, such as Providencia spp. These pathogens colonizing the urinary tract may transfer to other body organs, causing septicemia and subsequent organ-specific infections (1).
In contrast to most previous Providencia outbreaks, our patients primarily experienced VAP. Providencia species are not commonly found in endotracheal specimens; however, when detected, they are typically deemed to be colonizers rather than potential sources of infection. There are rare cases of VAP caused by Providencia species. Patel et al. reported a case of VAP caused by P. rettgeri in a postoperative man with prepyloric perforation, who survived with the timely administration of high-dose meropenem and cefepime (12). Sharma et al. reported two young men with VAP caused by P. stuartii, who expired despite antimicrobial susceptibility test-based treatment with aztreonam and minocycline (13). Interestingly, Pourheidar et al. recently reported an ICU-admitted case of VAP caused by PDR P. stuartii from our medical center, which survived after the administration of high-dose intravenous meropenem and amikacin, accompanied by nebulized amikacin (14).
According to the literature, most previously reported outbreaks of Providencia were caused by MDR or extensively drug-resistant (XDR) isolates (4, 15-19). Nevertheless, we encountered an outbreak in which all Providencia isolates were PDR. In the past, PDR Providencia species were uncommon, but their incidence has recently increased (18, 20). The emergence of PDR Providencia poses a significant health threat. Treating infections caused by Providencia spp. is difficult due to their intrinsic resistance to numerous commonly used antibiotics, such as ampicillin, first-generation cephalosporins, colistin, and tigecycline. Dealing with PDR pathogens is far more challenging and may result in a higher mortality rate (1) since there are fewer and occasionally ineffective antimicrobial agents available for treating these infections (19).
Many Providencia isolates can synthesize enzymes capable of breaking down carbapenems before they act on the bacteria, making them resistant to carbapenems. The emergence of extended-spectrum ß-lactamase (ESBL)- and carbapenemase-producing Providencia spp. should be considered a serious health challenge. These pathogens are also intrinsically resistant to polymyxins and tigecycline, which are the first therapeutic choices for treating carbapenem-resistant strains (19). Zavascki et al. reported the following three combination regimens for treating infections caused by carbapenem-resistant Providencia: Piperacillin/tazobactam (4.5 g every 6 hours) + meropenem (2 g every 8 hours), amikacin (1 g every day) + imipenem (500 mg every 6 hours), and levofloxacin (unavailable doses) (21). According to a study by Douka et al., piperacillin/tazobactam (4.5 g every 8 hours) combined with amikacin (1 g every day) was an effective therapy for PDR Providencia spp. (22).
Prolonged ICU hospitalization and administration of broad-spectrum antibiotics were the main risk factors for the PDR Providencia outbreak (23). Several data support the increasing antimicrobial resistance rate in many bacteria, primarily due to excessive antibiotic administration. Antimicrobial resistance can lead to treatment failure and increased cases of recurrent infections (19). The application of antimicrobial stewardship can reduce the length of ICU hospitalization and the antimicrobial resistance rate (24). Furthermore, transmission between patients and healthcare workers through hand colonization or environmental contamination might have contributed to the outbreak of PDR Providencia (6).
To eradicate the outbreak, we applied the following measures: Early detection of the outbreak, active screening of other patients, contact isolation precautions, extensive environmental disinfection with a 1% chlorine solution, and treatment with combined antimicrobial regimens. Consistent with our measures, previous outbreaks caused by Providencia spp. were also controlled with a multi-pronged approach, including an antibiotic stewardship program, universal precautions, and appropriate transmission-based precaution practices (19).
In this outbreak report, all initial and subsequent cultures from the ICU environment (ICU floor, ICU walls, tables, beds, and ventilators) were negative. Samples were obtained only from the surface of the ventilators. It is possible that ventilator filters were the source of infection. However, we have no sample and positive culture to prove this issue.
Moreover, most of the reported cases were elderly people with underlying diseases, which affected patient outcomes in addition to the resistance pattern of pathogens. Previous studies revealed that age, comorbidities, frailty, and physical dependence could worsen disease prognosis (25).
5.1. Conclusions
Clinicians should be aware of nosocomial infections caused by opportunistic pathogens, such as Providencia spp., especially in ICU-admitted patients with prolonged hospitalizations and broad-spectrum antibiotic administration. Strong clinical suspicion and timely detection of outbreaks, investigation of further cases, contact isolation of infected patients, disinfection of the environment, treatment with combined antibiotic regimens, and re-sampling of patients and the environment are the cornerstones of managing outbreaks caused by Providencia spp.