1. Background
Coronavirus disease 2019 (COVID-19) has become one of the deadliest pandemics in recent history (1). Although preventive measures such as physical distancing, wearing masks, remote work, isolation, and quarantine were introduced to slow the spread, these behavioral measures have had undesirable consequences, including negative psychological effects, deep depression, and other mental health issues (2). By the end of January 2020, there were 9,826 confirmed cases and 213 deaths in 27 countries. According to the WHO, as of October 17, 2023, 629,959,595 cases of coronavirus infection were recorded worldwide, with 6,571,489 deaths. In the Republic of Kazakhstan, 1,394,287 cases and 13,692 deaths were registered. In our country, 959 individuals are still receiving treatment for COVID-19 infection, with 107 in the hospital and 852 undergoing outpatient treatment. A total of 38,149 cases were recorded in Shymkent from 2020 to 2023.
Coronaviruses are a large family of viruses that can cause a range of diseases, from the common cold to more severe conditions such as middle east respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). In 2019, a new coronavirus (COVID-19) was discovered in Wuhan, China, which had never been detected in humans before (3). The development of a safe and effective vaccine emerged as the most promising goal in the fight against COVID-19 (3). Today, vaccination is one of the most effective tools to combat COVID-19 (4). However, studies on patients with various comorbid conditions have shown a lower immune response to vaccination (vaccine effectiveness) compared to the general healthy population (66.2% in patients with chronic diseases versus 93.4% in severe COVID-19 patients with multiple comorbid conditions) (5).
People with comorbidities have been disproportionately affected by COVID-19. Since regulatory trials for COVID-19 vaccines did not include individuals with immunodeficiencies, only a few cancer and autoimmune disease patients were involved. Given the limited available data on vaccine safety, vulnerable populations may have conflicting attitudes towards vaccines (5). Despite various hypotheses about a reduced immune response to vaccination, patients with comorbidities are still being vaccinated in the hope of developing an immune response to COVID-19. In the pre-vaccination era, serologic tests may have been used to evaluate the seroprevalence and effectiveness of containment strategies applied to the community. However, since the introduction of the SARS-CoV-2 vaccine, there has been a significant reduction in hospitalization rates and ICU admissions (6).
2. Objectives
This study aimed to compare the clinical and laboratory characteristics of vaccinated and unvaccinated patients hospitalized with COVID-19 pneumonia.
COVID-19 infection is considered one of the deadliest pandemics in recent times (1, 2). In 2019, a new coronavirus (COVID-19) was detected in Wuhan, China, which had never been identified in humans before. As of January 2020, there were 9,826 confirmed cases of the disease and 213 deaths across 27 countries. According to WHO data, as of October 17, 2023, there have been 629,959,595 confirmed cases of coronavirus infection worldwide, with 6,571,489 fatalities. In the Republic of Kazakhstan, 1,394,287 cases of illness and 13,692 deaths have been recorded. In Shymkent, 38,149 cases of coronavirus infection have been reported from 2020 to 2023 (3, 4). The development of a safe and effective vaccine is crucial for the successful management of COVID-19 (4). According to recent publications, results from patient cohorts with various comorbidities show a tendency toward a reduced immune response to vaccination (vaccine effectiveness), compared to the control group (66.2% in patients with chronic diseases versus 93.4% in severe COVID-19 cases with multiple comorbidities) (5). Comorbid patients have experienced more complications from COVID-19 than other population groups. Currently, there is limited reliable research on the effectiveness and safety of vaccines in these populations. Therefore, vulnerable groups may have conflicting attitudes towards vaccination (5). Despite various hypotheses regarding a low immune response to vaccination, patients with comorbidities should still be vaccinated to develop an immune response against COVID-19. Research has shown that vaccination against SARS-CoV-2 has successfully reduced the number of hospitalizations and intensive care unit admissions (6). This issue's significance has prompted us to study vaccinated patients against COVID-19. Our study examined the clinical and laboratory characteristics of patients with and without COVID-19 vaccination.
3. Methods
This retrospective study analyzed cases of patients who tested positive for COVID-19 by polymerase chain reaction (PCR) and were admitted to the City Infectious Diseases Hospital between January 2021 and October 2021. Approval for the study was obtained from the Clinical Research Ethics Committee of the South Kazakhstan Medical Academy (Decision no. 2021/0728, dated 12.01.2022).
Demographic data, including age, gender, smoking history, and recent health conditions, were recorded for all cases. Additionally, routine laboratory tests, clinical features, and COVID-19 vaccination status were documented for all patients diagnosed with COVID-19 pneumonia. Patients were categorized into two groups based on their COVID-19 vaccination status, and the clinical, radiological, and laboratory characteristics of both groups were compared.
Exclusion criteria included individuals who had received only one dose of the COVID-19 vaccine, those under 18 years of age, individuals with a negative PCR test for COVID-19, and pregnant women. The study focused on cases who were fully vaccinated with two doses of the vaccine, as per the standard protocol in our country at the time of the study.
Complete vaccination with the Sinovac-produced inactivated SARS-CoV-2 vaccine was defined as receiving two doses (0.5 mL) intramuscularly, with the last dose administered at least 14 days prior, or receiving two doses (0.3 mL) intramuscularly, with the last dose administered at least 21 days prior and not exceeding 6 months after the last dose. The vaccines included in the study were Pfizer, QazVac, Sinopharm/Beijing, Sinovac, and Sputnik V.
The required sample size for the study on COVID-19 vaccination in comorbid patients was determined using the Lehr formula for average values (with a study power of 80% and a significance level of 0.05). Substituting the values from a pilot study involving 16 patients, the minimum clinically significant frequency of complications (0.6), and the standard deviation (1), the minimum sample size for each group was calculated to be 75 people.
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software. Categorical data were presented as absolute values and percentages. Pearson's chi-squared test and Fisher's exact test were used to compare percentages in the analysis of two-by-two contingency tables, while Pearson's chi-squared test was used for multi-field contingency tables.
4. Results
The retrospective analysis included data from 510 cases admitted to a contagious conditions sanitarium between January 2021 and October 2021. In total, 510 cases were included in our study, divided into two groups based on their vaccination status: Those who had not been vaccinated against COVID-19 (n = 367) and those who had been vaccinated against COVID-19 (n = 143).
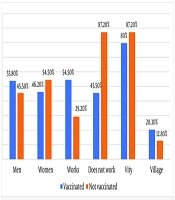
When comparing the two groups, the likelihood of non-working cases was higher in the unvaccinated group. The odds difference was statistically significant (OR = 0.577; 95% CI 0.347 – 0.961). The average age of the unvaccinated group was 57.66 ± 17.05 years, while the average age of the vaccinated group was 72.27 ± 14.08 years (P < 0.001). There were no significant differences in gender between the two groups. Comorbid conditions and cancer were more common in the vaccinated group.
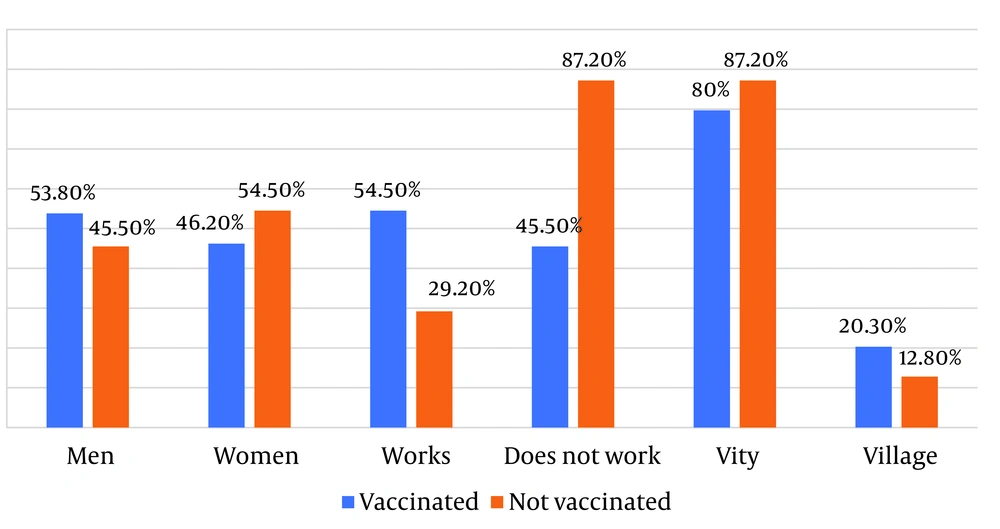
The duration of hospitalization in the unvaccinated group was 9.60 ± 6.0 days, whereas in the vaccinated group, the duration of hospitalization was significantly shorter at 7.19 ± 3.77 days (P < 0.001) (Figure 1). There was a statistically significant difference between the two groups in the number of hospitalizations to intensive care units (ICU), with 25.58% (n = 11/143) of vaccinated patients transferred to the ICU and 34.33% (n = 126/367) of unvaccinated patients transferred (P < 0.001). Statistically significantly lower values were also observed among the vaccinated patients for all analyzed characteristics (P < 0.001).
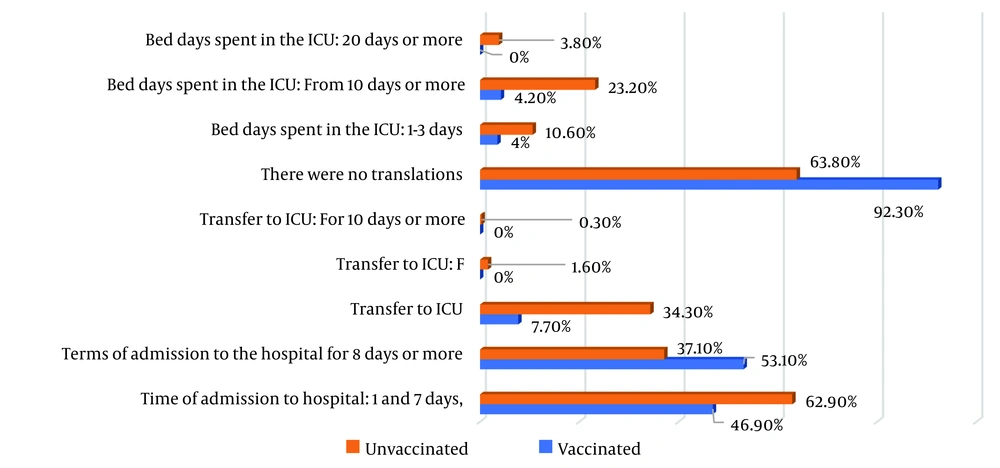
There was no statistically significant difference between the two groups in the number of complications (97.9%, n = 140/143 in the vaccinated group vs. 99.2%, n = 364/367 in the unvaccinated group). In terms of mortality, 9.8% (14/143) of cases died in the vaccinated group, compared to 21.3% (78/367) in the unvaccinated group (Figure 2). A statistically significant difference was observed between the two groups (P = 0.002).
According to the data obtained, when assessing the blood oxygen saturation index (SPO2) at admission, it was revealed that SPO2 < 68% was detected in 1 (0.7%) vaccinated patient and in 20 (5.4%) unvaccinated patients (P < 0.001) (Figure 3). In the study of biochemical parameters, the values of glucose and creatinine were statistically higher in unvaccinated patients (creatinine: Unvaccinated 9.8 ± 18.5). Serum levels of C-reactive protein (CRP) were 203 (55.3%) and procalcitonin (PRK) were 155 (42.2%) in unvaccinated patients; these levels were significantly higher (P = 0.005).
Additionally, serum levels of interleukin-6 (P < 0.001), troponin (P < 0.001), ferritin (P = 0.002), and D-dimer (P < 0.001) were significantly higher in unvaccinated patients (Table 1). According to computed tomography (CT) data, up to 50% lung damage was detected in 59.2% (n = 84) of vaccinated patients, whereas in the unvaccinated group, 75% or more lung damage was detected in 8.0% (n = 29) of patients (Table 2).
| Indicator | Vaccine Received a | Vaccine Not Received a | P-Value b |
|---|---|---|---|
| Ferritin | 27 (18.9) | 134 (36.5) | 0.002 |
| CRP | 25 (17.5) | 203 (55.3) | 0.004 |
| IHL procalcitoni | 41 (28.7) | 155 (42.2) | 0.005 |
| Interleukin-6 | 29 (20.3) | 135 (36.8) | < 0.001 |
| Fibrinogen | 17 (11.9) | 97 (26.4) | < 0.001 |
| D-dimer | 8 (5.6) | 95 (25.9) | < 0.001 |
| Troponin | 8 (5.6) | 103 (28.1) | < 0.001 |
| Glucose | 94 (65.7) | 284 (77.4) | 0.007 |
| Creatinine | 14 (9.8) | 68 (18.5) | 0.016 |
a Values are expressed as No. (%).
b The differences in the indicators are statistically significant (P < 0.05).
a Values are expressed as No. (%).
b The differences in the indicators are statistically significant (P < 0.05).
5. Discussion
In this study, we examined the importance of COVID-19 vaccination for individuals with underlying health conditions. Clinical trials of COVID-19 vaccines have demonstrated their safety and effectiveness in healthy individuals and have been extended to the adult population, prioritizing elderly individuals and those with underlying health conditions (7, 8).
Individuals who received the COVID-19 vaccine had a shorter recovery period, and their lung damage, as assessed by computed tomography, was less severe compared to those who did not receive the vaccine. Despite the high efficacy of vaccines demonstrated in trials and experimental studies, there remains a residual risk of serious COVID-19 complications, such as hospitalization or death, even after vaccination, although there is still time for immunity to develop (8).
Additionally, we observed differences in the immunological response, with vaccinated individuals showing higher levels of CRP and procalcitonin (PRK). Mortality rates were higher in the unvaccinated group, primarily due to older age and the presence of underlying health conditions such as cancer, diabetes mellitus, hypertension, and anemia.
As with other lung conditions, chest CT scans played a crucial role in assessing the severity of pneumonia caused by COVID-19 and monitoring response to treatment (9). Our study compared lung damage between COVID-19 patients who survived and those who did not, finding that lung damage was significantly lower in the vaccinated group. X-ray findings were similar between the vaccinated and unvaccinated groups, but there was significantly less limb involvement in the vaccinated group (9).
Our findings suggest that effective vaccination may mitigate the radiological progression of the disease. This hypothesis could be confirmed or refuted by studying antibody levels. Previous studies have shown that individuals with underlying health conditions have higher levels of cytokines in their blood, known as a cytokine storm (10). Our study confirmed elevated levels of interleukin-6, troponin, ferritin, and D-dimer in unvaccinated individuals. However, vaccinated individuals showed lower levels of inflammation.
It remains unclear whether this reduction in inflammation is due to a robust immune response triggered by vaccination or differences in the infecting virus strains. Nevertheless, vaccination significantly reduces hospitalizations and provides strong protection against severe illness and death, especially in adults over 65 years old (11).
The centers for disease control and prevention emphasize the importance of vaccinating individuals at high risk of severe illness (12). In a similar study, the characteristics of vaccinated and unvaccinated COVID-19 cases were compared (13). The unvaccinated group consisted of a younger population with fewer underlying health conditions compared to the vaccinated group. Vaccination was associated with reduced need for invasive mechanical ventilation and lower mortality rates.
In our study, both vaccinated and unvaccinated groups showed similar mortality rates and intensive care unit outcomes, possibly due to the small sample size. However, the vaccinated group included older individuals and those with more severe underlying conditions, yet still exhibited lower mortality rates, suggesting a protective effect of vaccination even in individuals with comorbidities (12, 14, 15).
Our data also suggest that individuals with underlying health conditions may have a lower immune response to vaccination compared to the general population. For example, low erythropoietin levels, associated with a weaker Th-1 response to antigens, were observed in the unvaccinated group, potentially leading to a less effective immune response (14, 16-20).
Overall, our findings support the efficacy of vaccination as a preventive measure against SARS-CoV-2 infections, particularly in individuals who develop mild to moderate COVID-19 illness after vaccination, as reported in other studies (18, 21-23).
5.1. Limitations
One of the primary limitations of our study is its retrospective nature. The lack of data on antibody levels in vaccinated individuals' blood, which are reliable indicators of immunity against COVID-19, is another significant limitation. Additionally, the study was unable to explore subgroups of vaccine administration, which could provide more detailed insights. Another limitation is the significant age difference between the two groups, which may have influenced the results. Furthermore, we did not examine the vaccination interval for COVID-19, which could also be an important factor. These limitations should be considered when interpreting the findings of this study.
5.2. Benefits
The advantage of this study lies in its multicenter design and the relatively large number of patients included, which enhances the generalizability and reliability of the findings.
5.3. Conclusions
Vaccinated individuals infected with COVID-19 had a shorter duration of hospitalization and less severe CT lesions. The levels of predictors for severe COVID-19, such as CRP, IL-6, D-dimer, and ferritin, were significantly higher in the unvaccinated group, indicating a more severe course of the disease. The mortality rate was also higher in the unvaccinated group compared to the vaccinated group. Despite the availability of COVID-19 vaccines to all Kazakhstanis, many people remain hesitant to get vaccinated. While current healthcare efforts to increase vaccine uptake have successfully reached many populations, additional efforts are needed to ensure that individuals with a history of COVID-19 are well-informed about vaccination recommendations. Furthermore, public education should focus on the possibility of re-infection and the role that unvaccinated individuals may play in the ongoing transmission of the virus, as well as the potential emergence of new variants of concern.