1. Background
Fasciola hepatica and Fasciola gigantica are major foodborne parasites responsible for fascioliasis, a serious and emerging infection affecting both humans and animals worldwide (1). Approximately 180 million people are at risk of Fasciola infection, with 2.4 to 17 million people currently infected globally (2, 3). Fascioliasis is recognized as a significant health concern in many countries, with its epidemiological patterns shifting as it emerges or re-emerges in various regions around the world (4). The global prevalence of fascioliasis among humans is increasing (1). Climate change has the potential to alter rainfall patterns and temperatures in various regions, creating new habitats suitable for Fasciola parasites and their hosts. Environmental modifications, such as irrigation projects, can also contribute to the availability of these habitats. These factors may lead to the expansion of endemic areas and an increase in the prevalence of human fascioliasis (5, 6). Iran, identified by the WHO as a significant endemic area for human Fasciola infection in Asia, is one of six countries facing serious challenges with fascioliasis. The infection is widespread among domestic animals in many parts of Iran, reaching up to 50% prevalence in some areas, with human cases reported in certain provinces, particularly in the north. Cases of human Fasciola infection have also been documented in other regions of the country (4). Zoonotic fascioliasis in Iran is influenced by factors such as the biology of the vector and parasite, livestock management practices, climatic conditions, drug resistance, food habits, ecological aspects of Fasciola transmission, the presence of an intermediate host, and free grazing by ruminants (7). Food habits, in particular, play a crucial role in determining the risk of fascioliasis. The consumption of wild watercress, which grows near livestock pasture areas, is implicated in most cases of sporadic transmission (5). The majority of sporadic Fasciola transmissions occur due to the consumption of wild watercress or other plants that grow near livestock pastures. Fascioliasis control strategies in endemic regions require fundamental information about various aspects of the disease, particularly its epidemiological patterns and transmission dynamics (8). Recent technological advances in Geographic Information Systems (GISs) have provided researchers with the ability to develop spatial and spatio-temporal risk maps for diseases (9). Geographic information system is invaluable for designing geographic distribution maps of disease prevalence, understanding factors that influence disease control and transmission, and identifying environmental variables that impact disease incidence (10).
2. Objectives
The primary objective of this research is to determine the incidence, spatial distribution, and hotspot regions of fascioliasis in Iran using GIS analysis for the period from 2013 to 2022.
3. Methods
3.1. Data Collection
Fascioliasis data from all provinces of Iran between 2013 and 2022 were obtained from the Ministry of Health (Disease Management Center), Tehran, Iran. This study was approved by the Research Ethics Committee of Kermanshah University of Medical Sciences (approval number: IR.KUMS.REC.1402.063). Meteorological data for the same 10-year period, including annual temperature and relative humidity for climatological terminals in 31 provinces, were acquired from the National Meteorological Organization.
The mean annual temperature and relative humidity for each province were calculated as follows: First, the average values for all weather stations within a province were determined, and then the mean of these averages across all stations within the province was computed. This process resulted in a single temperature value and a single humidity value for each province, which were used for subsequent analyses.
The normalized difference vegetation index (NDVI) layers for the month of May for the years 2013 to 2022 were calculated using Google Earth Engine software at a spatial resolution of one square kilometer per pixel. These layers were clipped in ArcMap based on provincial borders, and the mean NDVI value for each year and province was calculated. These derived NDVI values were utilized for the geographical weighted regression analysis in ArcMap 10.5.
The following formula was applied to calculate the incidence rate (per 100,000 population) for each province:
3.2. Spatial Analysis
The disease spatial database was constructed using ArcMap and included data on cases, patient age groups, locations, gender, and mortality across different age groups. Spatial distribution maps of the disease were generated for the period 2013 - 2022. Spatial analysis using ArcGIS 10.5 software, combined with the Hot Spot Analysis tool, was employed to identify disease hotspots across all provinces.
Hot Spot Analysis in ArcGIS is a suitable method for identifying provinces with significantly different disease incidences compared to others. This analysis provides Z-scores (both positive and negative) for each province, enabling the identification of significant disease hotspots and cold spots. A large positive Z-score indicates more critical points, whereas a higher negative Z-score indicates less critical points. The data points are classified into hot and cold spots at three confidence levels: 90%, 95%, and 99%, each associated with a specific Z-value (11).
Additionally, spatial analysis using geographically weighted regression (GWR) in ArcGIS 10.5 was conducted to explore the relationship between environmental variables (temperature, relative humidity, and NDVI density) and the incidence of fascioliasis from 2013 to 2022. Geographically weighted regression analysis allows for the modeling of spatially varying associations between the incidence of fascioliasis (dependent variable) and the average annual temperature, relative humidity, and NDVI (independent variables).
Statistical data analysis was performed using linear regression analysis and SPSS 21 software (Chicago, IL, USA), incorporating descriptive statistics tests.
4. Results
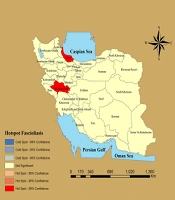
During the study period, 589 cases of fascioliasis were recorded in Iran. The incidence rate of human Fasciola infection is presented in Figure 1. The results revealed that the hotspots of the disease (areas with the highest incidence rates) were located in Gilan, Kermanshah, and Khorramabad provinces (Figures 2 and 3). Over the 10 years, the provinces of Gilan, Kermanshah, Khorramabad, Kurdistan, Semnan, and South Khorasan reported the highest rates of fascioliasis (Figures 4, 5, and 6).
The GWR analysis results for the period 2013 to 2021 demonstrated a strong correlation between humidity (Figure 4), temperature (Figure 5), NDVI (Figure 6), and the incidence of Fasciola infection in the provinces of Gilan, Kermanshah, Khorramabad, Kurdistan, Semnan, and South Khorasan. Data analysis showed that in 2022, there were 28 new cases of fascioliasis (including both males and females), with an infection incidence rate of 0.04 per 100,000 individuals.
The relationship between the incidence of fascioliasis (per 100,000 individuals), NDVI, relative humidity (RH), and mean temperature (TM) was analyzed using linear regression analysis (Table 1). The results demonstrated a significant relationship between humidity and NDVI with the incidence of fascioliasis.
| Province | TM | RH | NDVI | Incidence/100000 |
|---|---|---|---|---|
| Alborz | 16.40039214 | 35.1 | 0.226 | 0.015 |
| Ardabil | 9.268608143 | 69.3 | 0.386 | 0.024 |
| West Azerbaijan | 13.45255068 | 58 | 0.351 | 0 |
| East Azerbaijan | 12.74801269 | 49.95 | 0.325 | 0 |
| Bushehr | 25.58515797 | 61.65 | 0.107 | 0 |
| Chaharmahal and Bakhtiari | 12.47099015 | 44 | 0.266 | 0 |
| Esfahan | 14.02026325 | 38.85 | 0.115 | 0.006 |
| Fars | 17.68660851 | 41.45 | 0.154 | 0 |
| Gilan | 15.83153861 | 76.05 | 0.564 | 1.688 |
| Golestan | 18.53809255 | 67.9 | 0.404 | 0.006 |
| Hamedan | 10.10541655 | 48.55 | 0.31 | 0 |
| Hormozgan | 24.68627044 | 59.85 | 0.083 | 0 |
| Ilam | 20.31200242 | 41.05 | 0.16 | 0 |
| Kerman | 24.0960181 | 36.5 | 0.099 | 0 |
| Kermanshah | 15.59402365 | 40.15 | 0.335 | 0.106 |
| Khorasan Razavi | 15.07798998 | 47.1 | 0.145 | 0.008 |
| Khuzestan | 25.06462137 | 47.3 | 0.139 | 0.006 |
| Kordestan | 12.84783848 | 46.75 | 0.374 | 0.04 |
| Markazi | 12.21402871 | 43.8 | 0.223 | 0.021 |
| Mazandaran | 16.21690237 | 72.9 | 0.472 | 0.06 |
| North Khorasan | 14.71020272 | 56.3 | 0.215 | 0 |
| Qazvin | 15.22403958 | 52.55 | 0.282 | 0.008 |
| Qom | 16.56578786 | 47.85 | 0.104 | 0 |
| Semnan | 15.29186281 | 37.65 | 0.08 | 0.042 |
| Sistan Va Baluchestan | 23.32389757 | 31.75 | 0.071 | 0.008 |
| South Khorasan | 18.50197443 | 33.3 | 0.08 | 0.013 |
| Tehran | 15.81778573 | 36.6 | 0.161 | 0.04 |
| Yazd | 17.43841887 | 33.1 | 0.082 | 0 |
| Zanjan | 12.75088759 | 53.85 | 0.278 | 0 |
| Kohgiluyeh and Boyer Ahmad | 23. 07798998 | 40.65 | 0.218 | 0.03 |
| Khorramabad | 19. 53809255 | 43.6 | 0.312 | 0.2 |
Abbreviations: TM, temperature; RH, humidity; NDVI, normalized difference vegetation index.
According to the findings, an increase of one unit in relative humidity caused an increase of 0.009 in the incidence of Fasciola infection (P-value = 0.027). Similarly, an increase of one NDVI unit resulted in a 1.186 increase in the incidence of fascioliasis (P-value = 0.025) (Table 2).
| Variables | Univariate | Multiple | ||
|---|---|---|---|---|
| Beta (SE) | P-Value | Beta (SE) | P-Value | |
| TM | -0.003 (0.014) | 0.803 | 0.022 (0.015) | 0.152 |
| RH | 0.009 (0.004) | 0.027 | -0.001 (0.006) | 0.888 |
| NDVI | 1.186 (0.387) | 0.005 | 1.618 (0.677) | 0.025 |
Abbreviations: TM, temperature; RH, humidity; NDVI, normalized difference vegetation index.
5. Discussion
Geographic information system software plays a crucial role in epidemiological models for identifying risk factors involved in the transmission of fascioliasis and determining high-risk zones in previously unstudied areas (12). In this study, spatial analysis was utilized to estimate areas with high disease prevalence of fascioliasis. Interestingly, during 2013 - 2022, cold foci of the disease (provinces with significantly low disease incidence) were identified. The findings of this study align with the work of Malone et al. in the USA (13), which demonstrated that environmental factors such as air temperature, rainfall, and potential evapotranspiration are significantly related to the annual incidence of Fasciola infection in hosts. These environmental variables influence the free-living larval stages of eggs and metacercariae as well as snail (intermediate host) and parasite populations (14).
Moreover, Fuentes et al. applied GIS software to create a spatial and epidemiological model for mapping fascioliasis in the South American Andes. They categorized transmission risk into low, moderate, and high-risk areas, allowing them to pinpoint regions requiring targeted disease control measures (12). Notably, they employed modified climatic forecast indices (the MT and Wb-bs model) based on meteorological data from high-altitude and low-latitude endemic areas.
In our study, the GWR analysis showed a significant correlation between vegetation density, temperature, humidity, and the incidence of fascioliasis in Gilan, Kermanshah, Khorramabad, Kurdistan, Semnan, and South Khorasan (P-value = 0.025). Consequently, these provinces were identified as areas where fascioliasis infection is prevalent.
Regions with temperate climates, adequate rainfall, and favorable humidity provide optimal conditions for the parasite’s life cycle and the subsequent incidence of disease. Specifically, the provinces of Gilan, Kermanshah, Khorramabad, Kurdistan, Semnan, and South Khorasan exhibit relative humidity, average annual temperatures, and robust vegetation density, as confirmed through geographical weight regression analysis. These factors create an environment conducive to the breeding of the intermediate host, the survival of Fasciola larval stages, an effective parasite transmission cycle, and the contamination of both intermediate and final hosts (8, 15, 16). Consequently, fascioliasis incidence is notably higher in these areas.
The higher prevalence of Fasciola infection in these regions may also be attributed to the nomadic lifestyle of some populations, close contact with natural environments, the consumption of raw vegetables (particularly aquatic ones), and limited access to treated tap water (17). Infected ruminants act as major reservoirs for human infection due to their proximity to freshwater plants cultivated for human consumption. Furthermore, livestock breeding practices prevalent in these regions significantly influence the disease's transmission dynamics (16, 17).
Dietary habits play a significant role in the development of human Fasciola infection. Numerous wild aquatic and semi-aquatic plants have been identified as significant risk factors associated with human fascioliasis in Iran (18). These findings strongly suggest that while humidity and temperature are critical variables for the Fasciola parasite to complete its life cycle, other factors, such as vegetation density, also significantly affect fascioliasis prevalence and must be considered. In areas with varying environmental characteristics, diverse climate conditions significantly impact the development of snails as intermediate hosts for fascioliasis (19). Variables such as temperature, humidity, and vegetation density critically influence the survival of both the snail intermediate host and the larval stages of Fasciola trematodes.
Environmental temperatures above 10°C are essential for the development of the Fasciola parasite (miracidia and cercariae) and for snail reproduction. These stages are halted at temperatures below 5°C (19, 20). Optimal humidity conditions occur when rainfall exceeds transpiration, reaching saturation levels. These conditions are essential for miracidia to infect snails and for the dispersal of cercariae released from snails (20).
In our study, GWR analysis was employed to examine the correlation between temperature, relative humidity, and NDVI with the incidence of human fascioliasis. The results revealed a significant correlation between these variables and the incidence of fascioliasis in Gilan, Kermanshah, Khorramabad, Kurdistan, Semnan, and South Khorasan provinces (8, 15, 21).
The findings of Danson et al. further emphasized the value of GIS in studying human parasitic infections, providing enhanced insights into parasite transmission dynamics. Their research highlighted the impact of environmental factors such as temperature, humidity, and altitude on the distribution of worm infections (22), aligning with the findings of our study.
5.1. Conclusions
Geographic information system analysis can provide reliable predictions of Fasciola cases in endemic regions of Iran. Our study demonstrated a significant correlation between mean annual temperature, relative humidity, NDVI, and the incidence of fascioliasis in Iran. The data and results from this study can assist health policymakers in developing and sustaining prevention programs to address new cases. Accordingly, initiatives such as educating the population to avoid infection, screening participants who may act as reservoirs of fascioliasis, treating patients in endemic areas, and addressing environmental factors are crucial components of an effective control program.
Geographic information system, as an effective tool, is highly suitable for investigating and mapping the distribution of influencing factors in the health system. However, a limitation of our study was the presence of missing data during the data collection process.