1. Background
Calcium (Ca) is one of the key elements responsible for numerous functions in the body (1); its levels can be influenced by parameters such as parathyroid hormone (PTH), vitamin D, phosphorus, and magnesium levels. Any imbalance in these factors alters Ca levels. While hypercalcemia can be associated with increased PTH or vitamin D, conditions such as hypoparathyroidism, nephrosis, and pancreatitis can induce hypocalcemia (2). Moreover, vitamin D, as a fat-soluble vitamin, commonly enhances the absorption of Ca, phosphate, and magnesium, which are essential for the body’s functions (3).
The outbreak of COVID-19 was first identified in 2019 in Wuhan, China (4). Although fever, cough, and fatigue are the primary clinical manifestations, previous investigations have indicated the importance of assessing endocrine parameters in these patients (5-8). It has been noted that this novel virus is associated with abnormalities in various vitamins, electrolytes, and trace elements (9-11). In patients with COVID-19, hypocalcemia is one of the significant biochemical abnormalities and a common problem that may be linked to poor prognosis (12). Measuring ionized Ca levels in these patients revealed hypocalcemia in 82% of cases (13). Earlier studies have shown an association between vitamin D deficiency and viral conditions (14, 15). It has been suggested that vitamin D deficiency may affect infection and disease expression and that it plays a fundamental role in regulating the COVID-19 endocrine phenotype (16). Furthermore, vitamin D has a significant physiological role in maintaining appropriate immunity, reducing pro-inflammatory factors, and boosting anti-inflammatory cytokine production, which helps patients with COVID-19 (17). Despite speculation that COVID-19 might cause hypoparathyroidism (18), magnesium deficiency is widely considered to mimic the risk factors for its occurrence (18, 19).
Although the effect of viral infections on Ca metabolism is not a new concept, previous investigations focused on viruses such as SARS and Ebola during past outbreaks, highlighting hypocalcemia as a predominant biochemical abnormality (20, 21). It was even noted that 75% of patients affected by the Ebola virus had hypocalcemia during hospitalization (21).
2. Objectives
The present study, however, aims to assess Ca metabolism disorders in patients with COVID-19, a novel virus with distinct unknown aspects and complications. This study was conducted due to the scarcity of evidence on this issue and the importance of a thorough assessment. We hypothesize that our findings will shed light on this unexplored phenomenon.
3. Methods
3.1. Patients and Settings
This descriptive cross-sectional study was conducted in 2021 at Afzalipur Kerman Medical Center in Iran, focusing on patients hospitalized with COVID-19. The inclusion criteria consisted of individuals aged 18 to 60 years with a confirmed diagnosis of COVID-19 based on a positive PCR test, provided their clinical symptoms had manifested within the last 14 days. The exclusion criteria included a history of parathyroid diseases, chronic kidney disease, malignancies, known chronic medical conditions (such as cardiovascular, pulmonary, metabolic, rheumatological, hematological, neurological, or psychological disorders), pregnancy, breastfeeding, recent consumption of Ca and vitamin D supplements within the past month, and lack of consent to participate in the study.
In 2021, a total of 280 COVID-19 patients with positive test results were hospitalized at Afzalipur Hospital in Kerman. Using Cochran's sampling formula with an error level of d = 0.05, 162 of these patients were randomly selected to participate in this study.
3.2. Data Gathering
Data were collected in a format that included demographic characteristics and laboratory results.
3.3. Laboratory Tests
A 5 cc blood sample was obtained and collected in a clot test tube for the purpose of conducting various tests, including total Ca measurements using the photometric method with the Arsenazo III kit, ionized Ca assessments via the ISE method, magnesium level determination using the Xylidyl blue kit, intact parathyroid hormone (iPTH) analysis, and 25-OH-VITD3 measurement using the ELISA technique with the Monobind kit. The serum sample was carefully separated at the Afzalipur Hospital laboratory in Kerman and stored at -70°C. Subsequently, the sample was sent to the laboratory at Mehrgan Hospital in Kerman for further analysis.
The normal range is as follows:
Total calcium = 6.8 - 5.10 mg/dL
Ionized calcium = 1.12 - 1.32 mmol/L
Albumin = 5.3 - 2.5 g/dL
Magnesium = 8.1 - 6.2 mg/dL
iPTH = 15 - 65 pg/mL
25OHvitD ≤ 10 ng/mL: Deficiency
11 - 29 ng/mL: Insufficiency
30 ≤ ng/mL: Sufficiency
3.4. Ethics Approval and Consent to Participate
A written informed consent form was obtained from each patient, and they consented to participate in this study. This investigation was approved by the ethics committee of Kerman University of Medical Sciences (Code: IR.KMU.AH.REC.1400.216, Date: 2021-12-05). All experiments were conducted in accordance with the relevant guidelines and regulations of the Declaration of Helsinki.
3.5. Statistical Analysis
Data were gathered and reported as mean, standard deviation, number, and percentage using IBM SPSS version 21 software (SPSS Inc., Chicago, IL).
4. Results
The present study was conducted on 162 patients, comprising 59% males and 41% females, with a mean age of 49 years (Table 1).
| Variables | No. (%) |
|---|---|
| Gender | |
| Female | 67 (41) |
| Male | 95 (59) |
| Age groups (y) | |
| < 20 | 3 (2) |
| 21 - 30 | 11 (7) |
| 31 - 40 | 20 (12) |
| 41 - 50 | 26 (16) |
| 51 - 60 | 100 (62) |
| > 60 | 2 (1) |
| Underlying disease | |
| High blood pressure | 27 (17) |
| Diabetes | 43 (27) |
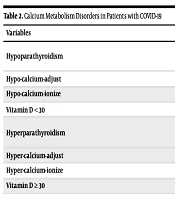
As indicated in Table 2, the most common Ca metabolism disorders in patients were low vitamin D, including deficiency and insufficiency (59%), hypercalcemia (43%), hypocalcemia (40%), vitamin D insufficiency (38%), hypomagnesemia (37%), and Ca adjustment abnormalities (35%). Moreover, the lowest prevalence of Ca metabolism disorders in these patients was hyperparathyroidism (7%) and Ca adjustment abnormalities (8%).
| Variables | No. (%) | Confidence Interval |
|---|---|---|
| Hypoparathyroidism | 40 (25) | |
| Ca | 7.3 (7.1 - 7.4) | |
| iPTH | 26.4 (20 - 33) | |
| Hypo-calcium-adjust | 56 (35) | 7.60 (7.5 - 7.7) |
| Hypo-calcium-ionize | 65 (40) | 0.92 (0.89 - 0.95) |
| Vitamin D < 30 | 97 (59) | 49 (46 - 51.8) |
| Hyperparathyroidism | 12 (7) | |
| Ca | 11.2 (10.5 - 11.8) | |
| iPTH | 42 (20 - 64) | |
| Hyper-calcium-adjust | 13 (8) | 11.6 (10.9 - 12.4) |
| Hyper-calcium-ionize | 69 (43) | 1.9 (1.7 - 2) |
| Vitamin D ≥ 30 | 65 (40) | 49 (46 - 51.8) |
5. Discussion
This research aimed to assess the prevalence of Ca metabolism disorders among patients hospitalized with COVID-19 at Afzalipur Kerman Medical Center. In this investigation, the most frequently observed Ca metabolism disorders among patients included low levels of vitamin D, encompassing both deficiency and insufficiency (59%), elevated ionized Ca (43%), diminished ionized Ca (40%), vitamin D insufficiency (38%), hypomagnesemia (37%), and reduced Ca adjustment (35%). Conversely, the least commonly found Ca metabolism disorders in these patients were hyperparathyroidism (7%) and excessive Ca adjustment (8%).
Depending on the definition of hypocalcemia, numerous studies have established a surprisingly high incidence of hypocalcemia, ranging from 62.6% to 87.2%. Studies that used total serum Ca levels to define hypocalcemia, such as 2.2 mmol/L (8.8 mg/dL), 2.15 mmol/L (8.6 mg/dL), or 2.12 mmol/L (8.5 mg/dL), observed hypocalcemia prevalence ranging from 62.6% to 74.7% (22-25). However, a higher prevalence (> 80%) was noted by those who defined it based on ionized serum Ca levels (12, 13, 26, 27). Although we found a consistent trend for hypocalcemia based on both total and ionized levels, our study showed lower levels of hypocalcemia (35% vs. 43%, respectively), which may be attributed to different sample sizes, measurement methods, and inclusion criteria for patients. Notably, Cappellini et al. observed lower total and ionized Ca levels in patients with positive nasopharyngeal swabs compared to patients admitted to emergency departments with similar signs and symptoms but negative nasopharyngeal tests (26). Although we included only patients with positive PCR results, and based on the above-mentioned investigation, higher hypocalcemia may have been expected, the findings still underscore the importance of measuring these parameters in patients with COVID-19.
The present work indicated that 59% of patients had vitamin D levels < 30, a high prevalence that has been confirmed by other similar investigations (22-25, 27-31). It is noteworthy that chronic hypovitaminosis D alters Ca metabolism and reduces the absorption of Ca and phosphorus from the intestinal tract. Additionally, COVID-19 may exacerbate hypocalcemia, particularly in those with pre-existing vitamin D deficiency and severe infections (29). However, there is ongoing debate regarding the treatment of vitamin D deficiency in patients with COVID-19 (32-35). The results of a systematic review and meta-analysis demonstrated that vitamin D supplementation decreased the risk of acute respiratory infections, primarily in individuals treated with daily or weekly doses, but not with bolus doses (36). In a single-center study, 36.7% and 86.7% of COVID-19 patients had hypocalcemia (Caadj < 2.15 mmol/L) and vitamin D deficiency (< 20 ng/mL), respectively. The study noted that these abnormalities were more common in patients with severe infections who required hospitalization, and they recommended further research to determine the effect of these impairments on improving treatment strategies (37). di Filippo et al., who retrospectively assessed 118 patients hospitalized for COVID-19, reported that 76.6% had hypocalcemia (total serum Ca < 2.2 mmol/L), with only 6.7% exhibiting hypervitaminosis D (> 30 ng/mL) (13). Moreover, Bossoni et al. reported a case of a hospitalized 72-year-old female with a positive nasopharyngeal swab for COVID-19. Her chief complaints for hospitalization were mild fever, headache, dysarthria, and perioral paresthesia. The patient was found to have total and ionized hypocalcemia, hyperphosphatemia, and hypoparathyroidism (38).
Although there are limited case reports on the effect of COVID-19 on the parathyroid gland, our results indicated that 25% of patients had hypoparathyroidism. A case report has presented COVID-19 as the cause of hypoparathyroidism in a 46-year-old male admitted for hypoxemia secondary to this viral infection, after excluding other known causes of hypoparathyroidism, such as genetic factors or malignancies. Due to the nature of their report, only limited conclusions could be drawn, but they emphasized the need for further studies to assess the link between COVID-19 and parathyroid dysfunction (39). Additionally, other case reports have noted this relationship (40, 41). These limited case reports, along with our substantial results, underscore the importance of a thorough understanding of the mechanisms through which COVID-19 may trigger parathyroid dysfunctions.
While normal magnesium levels exert a protective effect against viral infections, magnesium deficiency can be linked to these infections. Hypomagnesemia reduces the cytotoxicity of NK and T-cells, increases NF-κB expression, and promotes proinflammatory activities via the upregulation of cytokine production in monocytes (42). The results of the present investigation indicated that 60 patients (37% of all) had hypomagnesemia. Similarly, previous studies have highlighted the critical role of hypomagnesemia in the severe outcomes of COVID-19 infection. Quilliot et al., who assessed serum magnesium levels in 300 patients upon admission, demonstrated that 48% had hypomagnesemia. Furthermore, Guerrero-Romero et al. reported hypomagnesemia in 44.1% of patients (43, 44).
The first limitation of the present study is the absence of a control group. Further research can enhance the generalizability of the findings by addressing this limitation. Another limitation of this study is its single-center design, which may affect the results. This factor could not be controlled by the researchers, and thus it is recommended that multi-center studies be conducted to eliminate other confounding factors. Due to socio-economic differences across the country, similar research should be conducted in other regions of Iran, and the obtained results should be compared.
5.1. Conclusions
The results of this research indicated that there is a possibility of Ca metabolism disorders, especially hypocalcemia, in patients with COVID-19. Since this study was cross-sectional, it is recommended to conduct longitudinal and more comprehensive studies in this field for a more detailed investigation of the contributing factors and to establish a cause-and-effect relationship.