1. Introduction
Despite advances in diagnostic imaging and neurosurgery, brain abscesses continue to pose a significant threat to the central nervous system, although their mortality rate has markedly decreased compared to the past. Children, particularly those aged between 4 and 7 years, represent approximately one-fourth of all brain abscess cases (1, 2). Brain abscesses may develop from adjacent sources, including post-neurosurgical interventions, open traumatic brain injuries, sinusitis, mastoiditis, otitis media, or dental infections. They can also spread hematogenously, especially in individuals with pulmonary conditions or cyanotic congenital heart disease (CHD). In approximately 8% of cases, the origin of the abscess remains undetermined (3).
The mortality rates from brain abscesses have significantly decreased, from 30 - 60% in the late 20th century to 4 - 24% according to recent studies (1, 4, 5). This improvement is attributed to advancements in pediatric hygiene, updated vaccination practices, modern diagnostic imaging techniques, innovative neurosurgical methods, and the development of antibiotics effective against both aerobic and anaerobic bacteria (1, 4, 5).
The symptoms of brain abscesses in their initial stages can vary widely, presenting subtly or pronouncedly, with severity ranging from mild to critical. Factors influencing these symptoms include the patient's immune status, age, the progression, size, and location of the abscess, as well as the presence of concurrent meningitis (1, 5, 6). Contrary to the classic triad of fever, headache, and focal neurological deficits—observed in only 9 - 28% of younger patients (1, 5)—a change in consciousness level is more commonly noted (1, 5, 7).
Common risk factors for brain abscesses include neurosurgical procedures, dental issues, localized infections in the head or neck, and congenital heart defects (3, 8). Immunocompromised individuals are especially vulnerable to brain abscesses (1, 5, 7). While streptococci and staphylococci are commonly associated with these abscesses, 10 - 56% of cultured samples in studies report no bacterial growth (1, 5, 7, 9).
The primary treatment approach typically involves a combination of antimicrobial therapy, tailored to the identified organism or utilizing broad-spectrum antibiotics, alongside surgical intervention. However, exclusive reliance on antimicrobial treatment is preferred in cases of small lesions (under 2.5 cm), multiple abscesses, or abscesses located deep within the brain (1, 5, 6).
2. Case Presentation
A previously healthy five-year-old boy from a Syrian refugee camp in northern Jordan was transferred to our neurosurgical center from a primary healthcare clinic. Initially, he presented with fever and was diagnosed with bacterial tonsillitis at the camp clinic, where he received a five-day course of intravenous antibiotics. However, his condition worsened, with persistent fever, headaches, and recurrent vomiting.
Upon admission to our center, his symptoms included fever, worsening headaches, and vomiting. Hematological investigations revealed a white blood cell count of 19.47 × 103/mm3 with 92% neutrophils, hemoglobin of 12.80 g/dL, platelet count of 485.0 × 103/mm3, elevated ESR (85 mm/hr), and elevated CRP (20.62 mg/L). Other parameters, including renal and liver function tests, were within normal limits. A brain CT scan was performed, showing a deep-seated hypodense lesion in the left thalamus. Subsequent brain MRI characterized the lesion as ring-enhancing and diffusion-restricted, indicative of a brain abscess, with no significant edema or mass effect, and displaying intraventricular extension.
A detailed medical history revealed a possible foreign body aspiration event months earlier. A high-resolution chest CT scan identified a 0.8 × 0.6 × 0.4 cm foreign body in the right main bronchus, later confirmed to be a piece of nut, which was successfully removed via bronchoscopy (Figure 1).
Initial brain CT and MRI of the thalamic lesion, and chest CT-scan. A, initial axial non-enhanced brain CT scan; B, axial T1-weighted MRI with contrast showing a deep-seated, ring enhancing lesion within the left thalamus; C, axial and coronal chest CT scan, showing the foreign body in the right main bronchus.
The patient underwent frameless stereotactic aspiration of the abscess, yielding 12 cc of pus, which proceeded uneventfully and was followed by an excellent recovery. Microbiological evaluations, including aerobic and anaerobic blood cultures, Gram stain, acid-fast stain, fungal stains, urine culture, sputum culture, and pus cultures, were all negative. Stereotactic aspiration, coupled with a month-long regimen of broad-spectrum IV antibiotics, led to significant improvement in his clinical condition.
Upon admission, initial antibiotic therapy was started immediately with broad-spectrum intravenous antibiotics: Vancomycin 300 mg every 6 hours, ceftriaxone 750 mg every 12 hours, and metronidazole 250 mg every 8 hours. Given the negative pus culture results and the slow response in terms of persistent fever, ceftriaxone was switched to meropenem 600 mg every 8 hours. IV antibiotics were continued for 6 weeks, resulting in a favorable response, with fever resolution, improved headache symptoms, and excellent radiological findings.
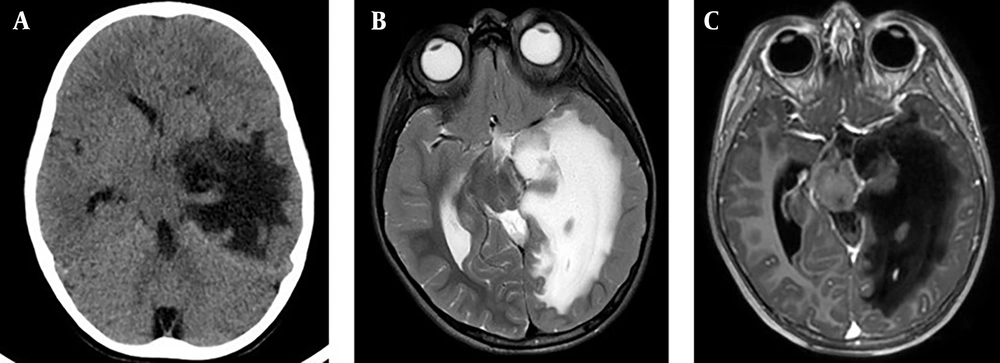
A follow-up brain CT scan and MRI performed one month post-surgery confirmed complete resolution of the abscess (Figure 2). However, on the 36th day of hospitalization, the patient developed sudden onset of right-sided weakness, aphasia, worsening headaches, and recurrent vomiting. An emergency brain CT scan and MRI revealed severe edema in the left temporal lobe, with a 1.5 cm midline shift and signs of uncal herniation (Figure 3).
Brain CT and MRI at day 36: Temporal lobe edema A, urgent brain CT on the 36th day of hospitalization, depicting massive temporal lobe edema with a midline shift and uncal herniation. B, T2-weighted MRI; C, axial T1-weighted MRI with contrast on the 36th day of hospitalization, depicting massive temporal lobe edema with a midline shift and uncal herniation, with no evidence of abscess recurrence.
Immediate medical management was initiated with administration of dexamethasone, mannitol, and Lasix to control brain edema. However, the patient’s clinical condition deteriorated rapidly, with a decline in consciousness that necessitated intubation, mechanical ventilation, and subsequent left decompressive craniectomy.
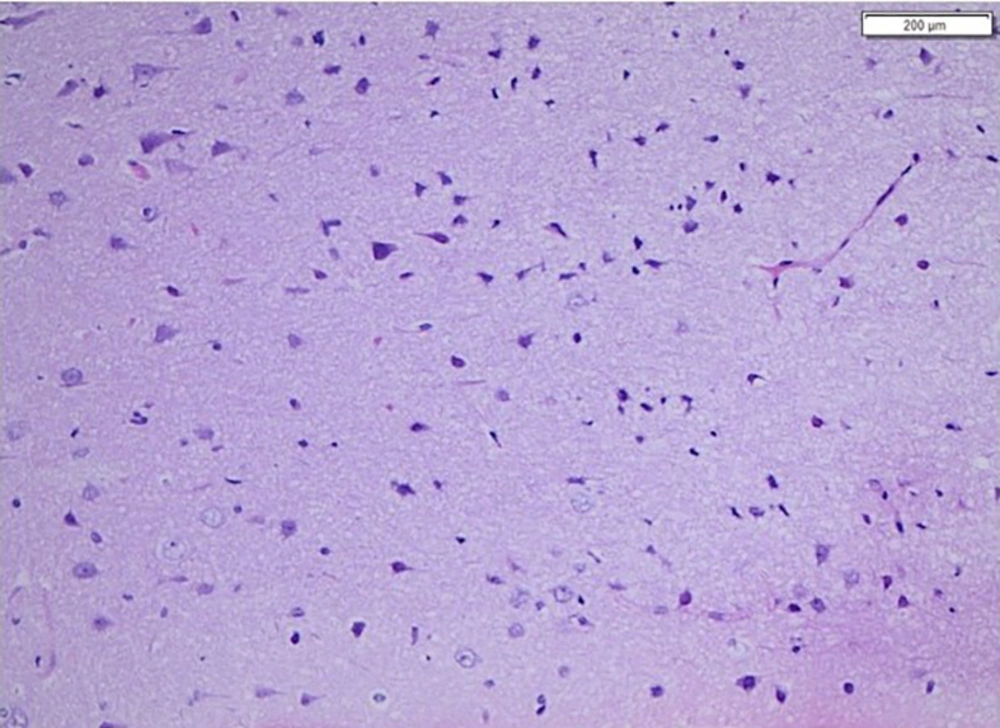
Further extensive investigations, including enhanced brain MRI, magnetic resonance angiography (MRA), and magnetic resonance venography (MRV), ruled out abscess recurrence, arterial or venous abnormalities, and cerebritis. Multiple cerebrospinal fluid (CSF) samples were analyzed and cultured, all yielding negative results. A brain biopsy was taken from the edematous area during the decompressive craniectomy. Histopathological examination ruled out cerebritis, autoimmune encephalitis, and other pathologies, and it was negative for infectious microorganisms on PAS special staining (Figure 4).
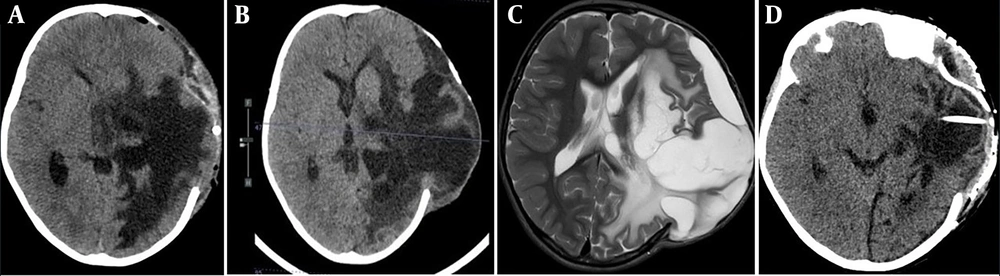
Despite all medical and surgical interventions, cerebral edema persisted, with progressive brain herniation through the craniectomy defect over the following 2 months. This situation necessitated the insertion of a cystoperitoneal shunt in the area of cystic encephalomalacia in the left temporal region, followed by cranioplasty. These procedures significantly improved brain herniation and led to a marked recovery in the patient’s right-sided weakness and aphasia (Figure 5), resulting in an excellent long-term functional outcome in terms of motor function, speech, and cognitive abilities.
CT and MRI post decompressive craniectomy; A, axial CT scan immediate post decompressive craniectomy; B, axial CT scan 1 month post decompressive craniectomy; C, axial T2-weighted MRI scan 2 months post decompressive craniectomy. Showing progression of the brain edema and herniation of brain tissue through the craniectomy defect; D, axial CT scan post cranioplasty and cystoperitoneal shunt insertion.
3. Discussion
This case presents a unique instance of idiopathic delayed brain edema (IDE) following the resolution of a brain abscess in an otherwise healthy five-year-old patient. Idiopathic delayed brain edema, an enigmatic and rarely reported condition, is typically asymptomatic in most cases, as documented in existing literature (10, 11). The distinctiveness of this case lies in the absence of arterial and venous anomalies and the delayed onset of brain edema after the resolution of the abscess, challenging our understanding of the pathophysiological mechanisms involved.
Brain edema is generally categorized into vasogenic and cytotoxic types (12). In this case, imaging results and the patient’s reversible clinical course suggest vasogenic edema as the likely cause. Vasogenic edema often arises from vascular damage, commonly following neurosurgical procedures, leading to a compromised blood-brain barrier (13). However, the delayed onset of edema in this case is atypical.
Differentiating IDE from other intracranial complications, such as intracerebral hemorrhage, arterial or venous infarction, and infections, is essential for appropriate management and prognosis (14-16). This case lacked typical symptoms of conditions such as hemorrhage or infarction (16) and had no systemic symptoms or positive microbiological cultures typically associated with infections (17, 18), which further complicated the diagnostic process.
The craniectomy performed to manage intracranial pressure introduced additional complexities, potentially exacerbating the external protrusion of the edematous brain (17-19). This underscores the challenges in managing IDE, particularly given the rapid progression observed in the subacute phase, which contrasts with the typical self-limiting nature of acute edema (20). We speculate that postoperative adhesions from an inflammatory response, coupled with a breach in the pia-arachnoid membrane post-decompressive hemicraniectomy, may have contributed to the sustained edema. However, the primary cause of IDE in this case remains unclear.
We propose two hypotheses for the delayed onset of IDE: An inflammatory response to microbial remnants from the resolved abscess, and a delayed cytokine-mediated autoimmune response targeting the brain parenchyma post-infection. Despite extensive investigations, these hypotheses remain speculative without substantial evidence.
The rarity and unexpected presentation of IDE in this case pose notable limitations. The lack of comparable cases in the literature restricts our ability to draw definitive conclusions. The diagnostic challenge is further compounded by the absence of positive microbiological cultures and clear pathological findings from tissue biopsies. While our hypotheses are shaped by the clinical progression and imaging results, they require further validation through ongoing research and additional case studies.
3.1. Conclusions
In conclusion, this case highlights the complexities involved in diagnosing and managing rare neurological conditions. It emphasizes the necessity of a multidisciplinary approach and continuous evaluation of treatment outcomes. This case provides valuable insights into the multifaceted nature of brain edema and its management, thereby enriching our understanding of neurological pathologies in pediatric patients.
This case underscores the complexities and unpredictability in managing pediatric brain abscesses, particularly when complicated by delayed cerebral edema. Our findings suggest that IDE can present in atypical ways, challenging established medical expectations and treatment protocols. The outcomes from this study emphasize the importance of vigilant postoperative monitoring and the potential for unusual presentations in pediatric neurological conditions. The implications of this case for pediatric neurology practice underscore the need for future research, including a registry of similar cases, to better understand the etiology and optimal management of IDE following brain abscesses in pediatric patients.
Although our study had limitations, such as the inability to definitively determine the etiology of IDE, the limited generalizability of its findings, and the speculative nature of its conclusions due to the idiopathic nature of the condition, it provides a foundation for future research into similar cases. We hope our study will aid in the development of more effective management strategies for similar cases in the future, thereby enhancing patient care and outcomes in pediatric neurology.
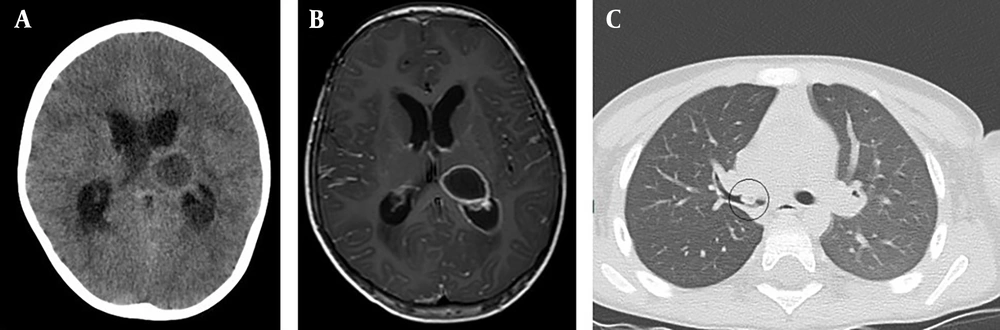
![Postoperative brain MRI and CT scan: Abscess Resolution. A-C, postoperative MRI scan 1 month after the initial surgery shows complete resolution of the brain abscess [A, T2; B, T1; C, DWI]; D, axial contrast-enhanced CT scan 1-month postsurgery showing complete resolution of the abscess. Postoperative brain MRI and CT scan: Abscess Resolution. A-C, postoperative MRI scan 1 month after the initial surgery shows complete resolution of the brain abscess [A, T2; B, T1; C, DWI]; D, axial contrast-enhanced CT scan 1-month postsurgery showing complete resolution of the abscess.](https://services.brieflands.com/cdn/serve/3170b/46d9453304de8390101aec3e48b93af82ea97c08/archcid-148927-g002-F2-preview.webp)