1. Background
Lyme disease, or Lyme borreliosis, is a significant zoonotic disease caused by the spirochete Borrelia burgdorferi and transmitted by ticks of the Ixodes family. Its incidence and geographic spread have progressively increased over the past few decades (1). The primary reservoirs for B. burgdorferi are small mammals (2). The B. burgdorferi sensu lato species complex includes three genospecies: B. burgdorferi sensu stricto, B. afzelii, and B. garinii, which are commonly associated with Lyme disease. The distribution and symptoms of the disease vary depending on the specific bacterial species involved. Borrelia species are obligate parasites with no free-living forms (3).
The life cycle of Borrelia alternates between two environments: Ticks and mammals or birds. Lyme Borrelia resides in the midgut of Ixodes ticks. During a blood meal, the spirochete population increases, and phenotypic changes, including the expression of outer surface protein C (OspC), occur. These changes enable the bacteria to invade the tick’s salivary glands (4). The OspC expression is also critical for establishing infection in mammalian hosts (3). While tick bites are the primary mode of transmission, direct contact with infected animal tissue or blood may also rarely cause transmission. This extracellular pathogen migrates through tissues, adheres to host cells, and evades immune clearance to cause infection (5). Although B. burgdorferi is typically considered an extracellular microorganism, it may adopt an intracellular form in nonphagocytic cells such as fibroblasts, potentially leading to immune escape or treatment failure (6).
Lyme disease progresses through three stages:
- Localized infection with skin manifestations.
- Disseminated infection occurring days to weeks later.
- Persistent infection, which may last for months to years.
The clinical presentation is variable. Some patients experience only localized skin infection, while others progress to later-stage manifestations, such as arthritis (3). The late-stage symptoms of Lyme borreliosis differ between the United States, Europe, and Asia. For example, an early U.S. study found that approximately 60% of untreated erythema migrans cases developed arthritis after an average of six months (7).
Lyme disease is most prevalent in the United States and Europe and is also frequently reported in Asia (8). A 2020 study by Naddaf et al. investigated the prevalence of hard ticks along Iran's Caspian Sea coast that were infected with Lyme borreliosis and relapsing fever Borrelia. In this study, Ixodes ricinus and other hard ticks were collected from various mammalian hosts, including sheep, goats, cows, camels, horses, dogs, donkeys, rodents, and poultry. Polymerase chain reaction (PCR) analysis of Borrelia 16S rRNA sequences revealed the presence of Borrelia in 71 of 501 samples from I. ricinus and Rhipicephalus ticks (9).
In endemic areas, the risk of human infection with B. burgdorferi is influenced by the prevalence and infestation levels of transmitting ticks and human behaviors that increase exposure. Activities like forestry work, hunting, and hiking are associated with a higher risk of infection (10). Lyme disease is highly endemic in northeastern and north-central United States, less common in central Europe, and has been reported in parts of Russia, China, Japan, Australia, India, Iran, Turkey, and North Africa. Data are limited in most African and Middle Eastern countries, and the disease appears to be rare in northern Canada and Russia. However, global warming may contribute to an increase in I. ricinus and I. persulcatus populations, altering the disease's geographic distribution (11). Iran is currently considered a non-endemic region for Lyme disease.
2. Objectives
Early diagnosis of Lyme disease is crucial to prevent severe complications. While tick bites are the primary mode of transmission, assessing infection prevalence in high-risk groups, such as slaughterhouse workers, is essential. However, there is a lack of recent regional studies on Lyme disease prevalence in such populations. Therefore, this study aimed to evaluate the prevalence of antibodies against B. burgdorferi in industrial slaughterhouse personnel.
3. Methods
This study is part of broader research evaluating various zoonotic infections among slaughterhouse workers in northeastern Iran. Serum sampling was conducted at the industrial slaughterhouse in Mashhad city, northeastern Iran, as previously described (12-14). Briefly, 91 samples (out of 450 workers) were included. Participants were randomly selected, and the sampling team collected blood samples over three days in the field. Required information, including demographic data and the use of personal protective equipment (PPE), was recorded in a predefined checklist. Informed consent was obtained from all participants before their inclusion in the study. The study was conducted in full compliance with the ethical principles of the Helsinki Declaration and received approval from the Ethics Committee of Mashhad University of Medical Sciences (ethical code IR.MUMS.fm.REC.1396.595).
3.1. ELISA Analysis
ELISA testing was performed on the serum samples from employees of the industrial slaughterhouse using an ELISA test kit (kit sensitivity = 96.6%, specificity = 95.2%, as stated in the kit manual). The Anti- B. burgdorferi VIsE ELISA (IgG) kit, produced by EUROIMMUN Company (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany), was used to detect IgG antibodies against B. burgdorferi. The ELISA procedure was carried out according to the manufacturer’s instructions. This kit employs the recombinant VIsE antigen of B. burgdorferi as the capture antigen for detecting antibodies in the serum. The VIsE antigen is a surface protein of B. burgdorferi, featuring conserved and highly immunogenic epitopes.
3.2. Statistical Methods
Statistical analysis was conducted using SPSS software. Group comparisons were performed using chi-square or ANOVA tests. Binary logistic regression was applied to evaluate odds ratios (OR). A significance level of 0.05 was used for all calculations.
4. Results
The demographic characteristics of the study participants are summarized in Table 1. Supplementary data provide details regarding the potential for occupational contact with animal diseases and the level of adherence to health practices by the participants. The mean age was 38.71 ± 8.07 years, ranging from 23 to 58 years. According to ELISA results, 10 out of 91 individuals (11%) tested positive. Based on the kit instructions, optical density (OD) values of 9 participants (9.9%) were classified as borderline, while 72 workers were in the negative range. The ANOVA test revealed no significant relationship between serological positivity and either the age or the duration of employment of the workers (P = 0.930 and P = 0.592, respectively).
| Variables | Frequency (%) |
|---|---|
| Gender | |
| Male | 91 (100) |
| Age (y) | |
| ≤ 40 | 47 (51.6) |
| > 40 | 44 (48.4) |
| Job | |
| Sheep butcher | 41 (45.1) |
| Cow butcher | 38 (41.8) |
| Administrative | 12 (13.2) |
| Role | |
| Participation in livestock slaughtering | 65 (71.7) |
| Abattoir inspection | 1 (1.3) |
| Transport and handling of livestock residues | 9 (11.3) |
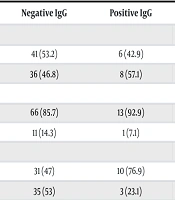
Additionally, the chi-square test showed no significant relationship between seropositivity and type of occupation, use of PPE, or direct contact with animal viscera (P > 0.05). However, eight individuals (19.5%) who worked with sheep tested positive, compared to only one seropositive individual (2.6%) who worked with cattle, indicating that working with sheep was significantly associated with increased seropositivity [chi-square test: P = 0.03; OR = 8.97; 95% CI (1.06 - 75.56)].
Table 2 presents the relationship between seropositivity and the occupational characteristics of the participants. As noted, the type of animal contact had a significant association with the antibody test response. Table 3 illustrates the relationship between the antibody test response and factors related to exposure to animal diseases and adherence to hygiene practices among participants. Although none of the evaluated factors showed a statistically significant correlation with the antibody test response (P > 0.05), all seropositive participants had contact with animal viscera more than once a week, though this association was not statistically significant.
| Variables | Negative IgG | Positive IgG | P-Valueb |
| Age (y) | 0.567 | ||
| ≤ 40 | 41 (53.2) | 6 (42.9) | |
| > 40 | 36 (46.8) | 8 (57.1) | |
| Job | 0.467 | ||
| Butchery | 66 (85.7) | 13 (92.9) | |
| Administrative | 11 (14.3) | 1 (7.1) | |
| Type of livestock | 0.048 | ||
| Sheep | 31 (47) | 10 (76.9) | |
| Cow | 35 (53) | 3 (23.1) | |
| Type of work | 0.262 | ||
| Slaughtering of livestock or livestock visceral disposal | 61 (91) | 13 (100) | |
| Other | 6 (9) | 0 (0) |
a Values are expressed as No. (%).
b The chi-square test has been used to compare between two groups.
| Properties and Times | Negative IgG | Positive IgG | P-Value |
|---|---|---|---|
| Contact with animal viscera | 0.240 b | ||
| More than 1 time per week | 70 (90.9) | 14 (100) | |
| Less than 1 time per week | 7 (9.1) | 0 (0) | |
| History of hand cutting over a year | 0.154 b | ||
| > 5 times | 48 (63.2) | 6 (42.9) | |
| ≤ 5 times | 28 (36.8) | 8 (57.1) | |
| External parasite infestation over a year | 0.984 b | ||
| > 5 times | 27 (36) | 5 (35.7) | |
| ≤ 5 times | 48 (64) | 9 (64.3) | |
| Use of mask | 0.335 b | ||
| Always | 28 (36.4) | 7 (50) | |
| Sometimes, seldom, never | 49 (63.6) | 7 (50) | |
| Use of gloves | > 0.999 c | ||
| Always | 72 (96) | 14 (100) | |
| Sometimes, seldom, never | 3 (4) | 0 (0) | |
| Use of gowns and aprons | > 0.999 c | ||
| Yes | 73 (94.8) | 14 (100) | |
| No | 4 (5.2) | 0 (0) | |
| Use of work boots | > 0.999 c | ||
| Yes | 73 (94.8) | 14 (100) | |
| No | 4 (5.2) | 0 (0) | |
| The amount of use of PPE (including masks, gloves, gowns and boots) | 0.335 b | ||
| Complete (use of all 4 items) | 28 (36.4) | 7 (50) | |
| Relative (use 3 or less) | 49 (63.6) | 7 (50) | |
| Disinfection rate of work tools | 0.784 b | ||
| Always | 9 (11.7) | 2 (14.3) | |
| Seldom | 68 (88.3) | 12 (85.7) | |
| Hand and face disinfection rate | 0.406 b | ||
| Always | 10 (13) | 3 (21.4) | |
| Seldom | 67 (87) | 11 (78.6) |
Abbreviation: PPE, personal protective equipment.
a Values are expressed as No. (%).
b The chi-square test has been used to compare between two groups.
c The Fisher's exact test has been used to compare between two groups.
5. Discussion
In this study, the prevalence of anti- B. burgdorferi IgG antibodies was assessed among personnel of an industrial slaughterhouse, with 91 individuals participating. The findings revealed that 11% of participants tested positive for anti- B. burgdorferi IgG antibodies. A 2022 systematic review and meta-analysis reported an average global prevalence of B. burgdorferi antibodies at 14.5% (15). In Iran, no recent reliable studies have examined the prevalence of these antibodies in the general population, limiting the ability to compare our findings with the national prevalence.
The results indicated no significant correlation between age, type of work at the slaughterhouse, and antibody test results. However, there was a notable association between the type of animal contact and antibody test responses. Among those who tested negative, 47% worked with sheep and 53% worked with cattle, whereas among those with positive antibody results, 76% worked with sheep and only 23% worked with cattle. This suggests a higher prevalence of antibodies among individuals who worked with sheep compared to those working with cattle. A 2021 study in Egypt examining 100 cattle, camels, and dogs found that while over three-quarters of the cattle were infested with ticks, no Borrelia cases were detected in the cattle (16).
Further analysis in our study showed that all seropositive individuals had close contact with animal viscera more than once a week, although this relationship was not statistically significant. As noted, B. burgdorferi transmission through contact with the blood and viscera of infected animals is rare (17).
Additionally, there was no significant relationship between the use of PPE, such as gloves, gowns, boots, and masks, and antibody test results. Similarly, adherence to hygiene practices, such as routine disinfection of hands and work tools, was not correlated with positive antibody results.
Data regarding B. burgdorferi in Iran is generally scarce. The detection technique is not routinely employed by clinical laboratories, which may lead to underdiagnosis of the infection. However, there are reports confirming the presence of the disease in Iran. For instance, cases have been confirmed in Isfahan, located in central Iran (18). Additional studies have identified infections in Tehran (19, 20) and Mazandaran (21). A meta-analysis has even classified Lyme disease as an emerging infection in Iran (22), with rare presentations such as neuroborreliosis also reported in the country (23). Moreover, veterinary research in Iran has focused on detecting B. burgdorferi in animals (24-27) and ticks (9, 28), further highlighting the potential for human infection, particularly among high-risk groups.
None of the seropositive individuals in our study reported typical Lyme migratory erythema. However, it is important to note that not all infections with B. burgdorferi manifest with typical erythema. Outcomes can range from asymptomatic cases (29) to non-specific arthritis (30) or neurologic manifestations (23, 31). Therefore, relying solely on the presence of a typical rash to diagnose the infection can be misleading.
The infection is prevalent across a geographical belt stretching from Asia to Europe and North America (32), including the Middle East (32, 33). However, its frequency may be underestimated due to the unavailability of standard serological diagnostic facilities.
In a 2020 study, Obaidat et al. examined the prevalence of anti- B. burgdorferi antibodies in the Jordanian population. Serum samples from 824 healthy individuals from various regions of Jordan were collected and tested. The results indicated that 11.7% of participants were positive for these antibodies (34). Similarly, Brummitt et al., in a study published in 2020, investigated the prevalence of anti- B. burgdorferi and Borrelia miyamotoi antibodies in blood donors in California, USA. They analyzed 1,700 blood samples using ELISA and confirmed positive cases with a Western blot test. The findings showed that only 0.47% of individuals tested positive for anti- B. burgdorferi antibodies (35).
The prevalence of anti- B. burgdorferi antibodies in our study (11%) was significantly higher than the prevalence reported by Brummitt et al. (35). This difference may be attributed to variations in geographic regions, technical methodologies, and study populations. Blood donors typically represent the general population, while our study group comprised slaughterhouse personnel, a high-risk population with frequent exposure to animals, increasing their likelihood of exposure to the pathogen.
Our study holds important epidemiological and clinical implications. Future research should focus on investigating the prevalence of Borrelia antibodies in diverse populations. A limitation of our study was the relatively small sample size and the absence of data on the general population, such as blood donors. Additionally, the study relied solely on ELISA for laboratory analysis without confirmatory testing using the Western blot technique. While the ELISA kit manual indicated high specificity, the potential for cross-reactivity cannot be ruled out (36). Therefore, this study serves as a preliminary investigation and highlights the need for further research.
Despite these limitations, our study's strength lies in being the first to examine anti- B. burgdorferi IgG antibodies in slaughterhouse workers in Iran. Future studies should explore the prevalence of anti-Borrelia antibodies in the general population, including blood donors and other high-risk groups. Moreover, periodic studies should be conducted to monitor trends and changes in the prevalence of the disease in both general and high-risk populations.
5.1. Conclusions
Approximately 11% of slaughterhouse workers tested positive for B. burgdorferi antibodies, with the majority of these individuals frequently handling sheep. All individuals with positive antibody tests had contact with animal viscera more than once per week; however, this association was not statistically significant. Based on our literature review, no study to date has investigated the prevalence of B. burgdorferi antibodies in the Iranian population. The present study serves as a health warning for high-risk occupations, emphasizing the need for full diagnostic evaluations in cases of clinical suspicion.