1. Background
Oral health is essential for overall well-being, with a balanced oral microbiome playing a crucial role in preventing dental diseases like caries and periodontal infections (1-4). Among various oral pathogens, Candida albicans (C. albicans) and Streptococcus sanguinis (S. sanguinis) are notable due to their roles in oral candidiasis and dental plaque formation, respectively (1, 2, 4-7).
Oral candidiasis, primarily caused by C. albicans, is a common opportunistic fungal infection. Candida albicans is a natural component of the oral flora, existing in both yeast and hyphal forms (8-10), and can cause mucosal infections in the mouth as well as systemic infections in immunocompromised individuals (1, 5, 6). In contrast, S. sanguinis contributes significantly to dental plaque formation and is implicated in endocarditis, dental caries, and periodontitis (2).
Antimicrobial mouthwashes are commonly used alongside mechanical oral hygiene practices to control these microorganisms (11-13). Chlorhexidine (CHX) is a well-established antimicrobial agent valued for its broad-spectrum activity and low toxicity, making it a staple in dental care (14-16). Storhaug conducted a pioneering study in 1977, demonstrating that CHX significantly reduces microbial plaque and gingivitis (17). However, CHX use has limitations, such as tooth discoloration, altered taste perception, and disruption of the oral microbiome (16).
Fluorine Total (FT) mouthwash, containing cetylpyridinium chloride (CPC) and fluoride, offers a potential alternative with fewer adverse effects. This formulation provides multiple benefits, including antibacterial properties, elimination of bad breath, increased enamel resistance, and prevention of early dental caries (11, 18, 19). Cetylpyridinium chloride, in particular, shows strong antibacterial properties against gram-positive bacteria like S. sanguinis. It binds to bacterial cell walls, disrupting their membranes, causing cytoplasmic leakage, and inhibiting metabolism and proliferation, in a manner similar to CHX (19, 20).
In addition to its antibacterial effects, CPC possesses antifungal properties. Some studies suggest that it may be more effective than common antifungal agents, such as miconazole, in preventing the adhesion and colonization of fungal pathogens like Candida on mucosal surfaces, which makes it particularly useful in managing oral candidiasis (21, 22). Moreover, mouthwashes containing CPC tend to cause fewer adverse effects, such as reduced tooth staining, and provide improved user satisfaction due to a more pleasant taste compared to CHX (23, 24).
2. Objectives
Although FT mouthwash may offer potential benefits, research on its effectiveness remains limited, especially in comparison to CHX. This study aimed to compare the effects of FT and CHX mouthwashes on the growth rates of C. albicans and S. sanguinis, providing a comprehensive evaluation of FT’s efficacy and its potential role in oral health care.
3. Methods
3.1. Study Design and Setting
This laboratory-based experimental study aimed to compare the antimicrobial efficacy of CHX and FT mouthwashes against C. albicans and S. sanguinis. The research was conducted in a microbiology laboratory setting.
3.2. Sample Size
A total of 40 samples were analyzed, comprising 18 plates for each microorganism (9 for CHX and 9 for FT) and 4 control samples. The sample size was determined using a two-sample t-test power analysis with a significance level of α = 0.05 and a power of β = 0.80, based on previous studies (11).
3.3. Inclusion and Exclusion Criteria
3.3.1. Inclusion Criteria
Samples were collected from individuals aged 18 - 65 who presented with oral health concerns.
3.3.2. Exclusion Criteria
Samples from individuals who had taken antibiotics within the past month or had a known fungal infection were excluded from the study.
3.4. Sample Preparation
3.4.1. Candida albicans
Candida albicans samples were prepared using a 0.5 McFarland suspension of ATCC 10237 C. albicans (equivalent to 0.5 × 106 CFU/mL). The samples were cultured onto 18 Sabouraud Dextrose Agar (SDA) plates, each with two wells, using the spread method. Nine plates were treated with 0.2% CHX (Behsa Company), and 9 with FT (FT mouthwash contains 0.05% sodium fluoride and 0.07% CPC, produced by Azad University, Pharmaceutical Sciences Unit).
The SDA medium was prepared by dissolving 41 gr of SDA powder (Sigma Aldrich) in 1 L of sterile distilled water, bringing it to a boil, autoclaving at 121°C under 1.5 atmospheres of pressure, and pouring it into 10 cm plates.
3.4.2. Streptococcus sanguinis
Streptococcus sanguinis samples were prepared using a 1.5 McFarland suspension of ATCC 10556 (equivalent to 1.5 × 108 CFU/mL), cultured on 18 blood agar plates, each with two wells, and incubated at 37°C for 24 hours.
The blood agar medium was prepared by dissolving 40 grams of blood agar powder (Sigma Aldrich) in one liter of sterile distilled water, bringing it to a boil, autoclaving at 121°C under 1.5 atmospheres of pressure, and pouring it into 10 cm plates. After the medium had cooled, 5% fresh sheep blood was added.
3.4.3. Positive Control Samples
For C. albicans, cultures were grown on SDA without the addition of any antifungal agents. Similarly, for S. sanguinis, cultures were grown on blood agar without the presence of any antibacterial agents.
3.4.4. Negative Control Samples
For C. albicans, cultures were exposed to a solution prepared from 400 International Units of Nystatin (provided by Emad Company) using the well diffusion method. Similarly, for S. sanguinis, cultures were treated with a solution prepared from 100 International Units of Penicillin (sourced from Padtan Teb Company) using the well diffusion method.
3.5. Growth Inhibition Zone Evaluation
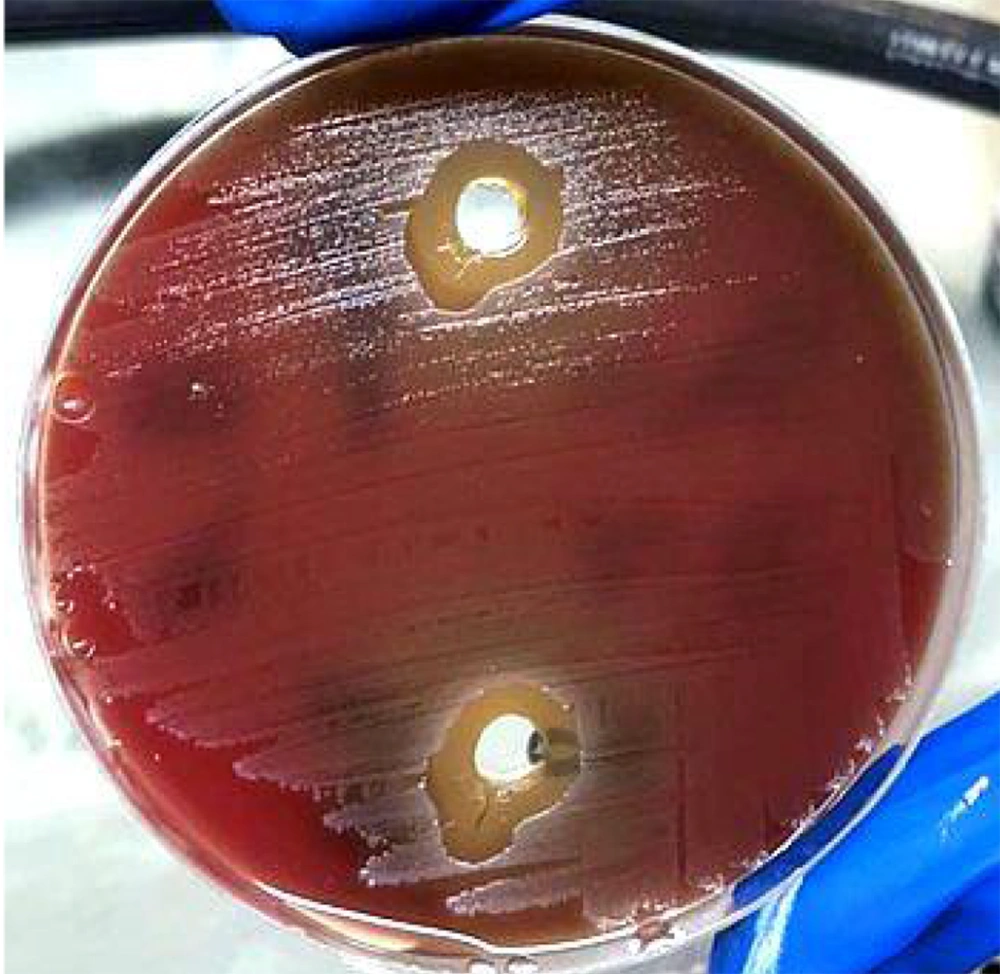
The wells of each plate were filled with CHX and FT mouthwashes. After a 24-hour incubation period at 37°C, the diameter of the growth inhibition zones surrounding the treatment wells was measured twice with a ruler to ensure accuracy and precision.
3.6. Data Analysis
All data were analyzed using SPSS version 25. The Mann-Whitney U test was employed for data that did not follow a normal distribution, while a two-tailed independent t-test was applied for normally distributed variables. Statistical significance was defined as P < 0.05.
4. Results
4.1. Candida albicans
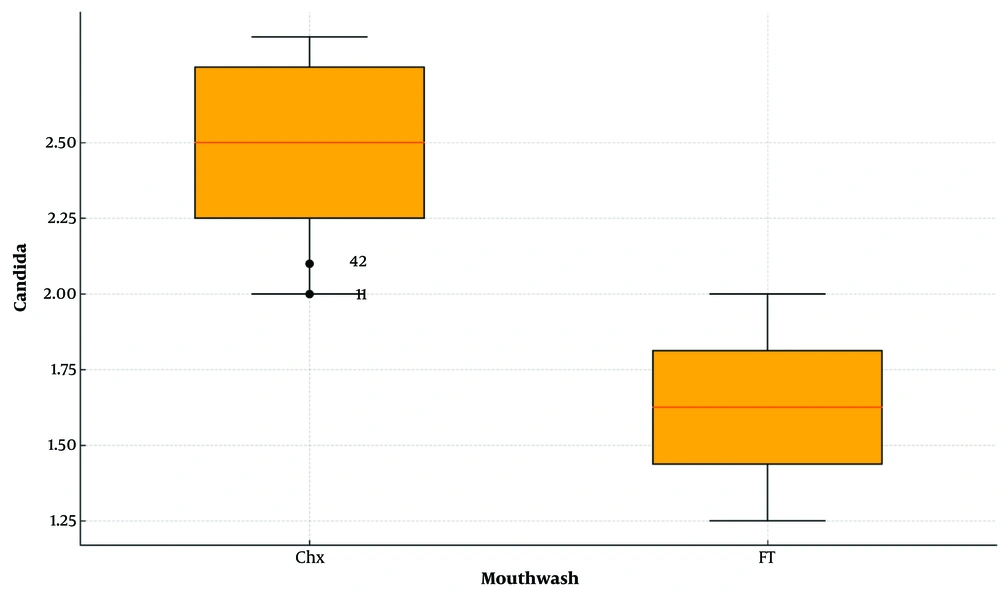
The mean diameter of the growth inhibition zone for C. albicans was 2.41 ± 0.17 mm with CHX treatment, compared to 1.47 ± 0.08 mm with FT. The distribution of inhibition zone diameters for C. albicans was not normally distributed (P < 0.05, Kolmogorov-Smirnov test). Consequently, the Mann-Whitney U test was applied to compare the inhibition zones between the two groups. The test indicated a statistically significant difference, with CHX showing a significantly larger inhibition zone than FT (P < 0.001) (Figure 1).
4.2. Streptococcus sanguinis
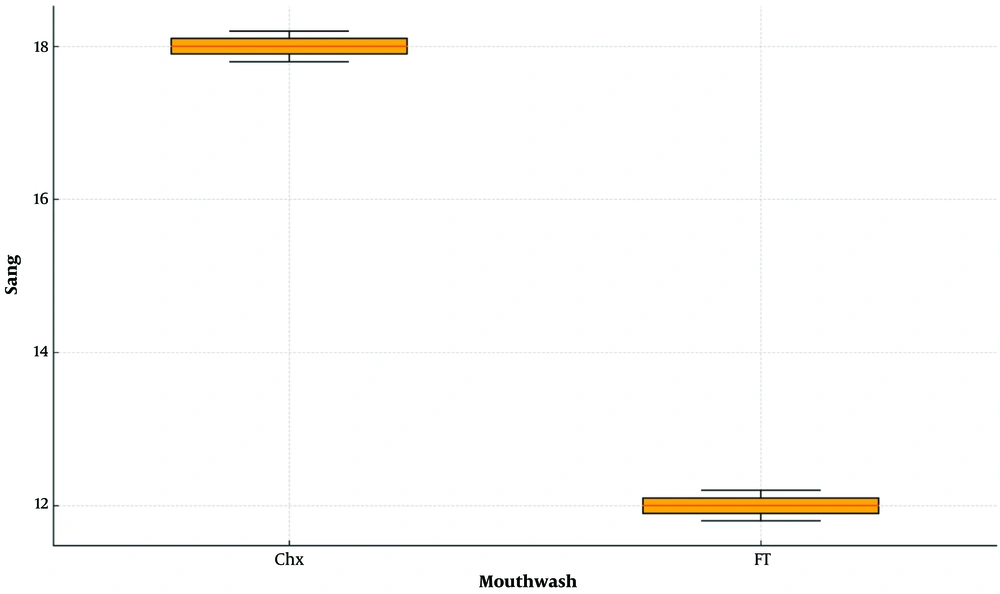
For S. sanguinis, the mean diameter of the growth inhibition zone was 17.96 ± 0.11 mm with CHX treatment, compared to 12.13 ± 0.18 mm with FT. As the data for S. sanguinis inhibition zones followed a normal distribution, a two-tailed independent t-test was used. The test revealed that CHX produced a significantly larger inhibition zone than FT (P < 0.001) (Figure 2 and 3).
5. Discussion
This study aimed to evaluate and compare the antimicrobial efficacy of CHX and FT mouthwashes against C. albicans and S. sanguinis by measuring the diameters of growth inhibition zones. The findings revealed that CHX exhibited significantly greater inhibitory effects on both C. albicans and S. sanguinis compared to FT.
Our results align with previous studies, such as Farrokhnia et al. (11), which also found that CHX mouthwash displayed superior antibacterial effects against Streptococcus mutans compared to FT. Although their study focused on a different bacterial strain, the consistent findings across various microorganisms reinforce the reliability of CHX's broad-spectrum antimicrobial properties.
Furthermore, studies like Ardizzoni et al. (25) have demonstrated the strong antifungal activity of CHX against C. albicans, supporting our findings. The superior efficacy of CHX is likely due to its strong binding affinity to microbial cell membranes, which disrupts membrane integrity, increases permeability, and causes intracellular leakage, ultimately inhibiting cell function and proliferation.
Evans et al. (26) also studied the inhibitory effects of various antiseptic mouthwashes, including CHX, on S. sanguinis, S. mutans, and Lactobacillus acidophilus. Among the mouthwashes tested, CHX, CPC, povidone, and sodium hypochlorite proved the most effective. This study aligns with the current research by examining the effects of both CPC and CHX on S. sanguinis, further validating CHX’s superior antimicrobial performance.
Furthermore, a recent study by Jain et al. (27) observed the antimicrobial effects of CHX on C. albicans and S. mutans, reporting inhibition zones of 12.4 mm for C. albicans and 20.85 mm for S. mutans. These results align with our findings, which indicate CHX’s effectiveness against both tested organisms, with particularly high efficacy against bacterial samples.
A 2020 study by Souza et al. (28) examined the prevalence of various oral Candida species in cancer patients and compared the effects of different mouthwashes. While both 5% cetylpyridinium and 0.12% CHX mouthwashes showed acceptable effects against C. albicans, Souza et al. found 5% cetylpyridinium to be more effective, a result contrasting with our findings. This discrepancy may stem from the lower CHX concentration used in Souza et al.'s study and possible variations in the specific Candida strains examined.
Chlorhexidine outperforms FT mouthwash due to its superior mechanism of action and broader spectrum of activity (11, 23, 24, 27). While FT primarily relies on CPC for its antimicrobial properties—effective against certain gram-positive bacteria and exhibiting limited antifungal activity—it does not match CHX's extensive efficacy (11, 29-31). Chlorhexidine’s mechanism involves robust adhesion to microbial membranes, significantly disrupting cell integrity and increasing membrane permeability. This action leads to osmotic imbalance and extensive leakage of intracellular contents, making CHX effective against both gram-positive and gram-negative bacteria, as well as fungi. Additionally, CHX’s strong binding within the oral cavity provides a prolonged antimicrobial effect, ensuring a sustained protective environment. By comparison, CPC’s antimicrobial effects are more limited and less durable, positioning CHX as a more comprehensive solution for maintaining oral hygiene (30-32).
Despite the strengths of this study, it has a few limitations. First, as a laboratory-based study, it does not account for real-world factors such as retention time in the oral cavity, salivary flow, and interactions with food particles. Second, the study only examined the immediate antimicrobial effects and did not assess the long-term impact of the mouthwashes on oral health. Future studies should include clinical trials to evaluate the effectiveness of these mouthwashes in actual patient populations.
5.1. Conclusions
Based on the findings of this study, CHX mouthwash demonstrated superior efficacy against both C. albicans and S. sanguinis compared to FT mouthwash. For therapeutic purposes, CHX is recommended, while FT may be suitable for daily and adjunctive use. However, further clinical research is needed to validate these results in real-world settings.