1. Background
Infections, despite advancements in transplant surgery and immunosuppressive medication, remain a significant cause of morbidity and mortality after liver and kidney transplantation, surpassing even acute rejection as the most common cause of disability and death (1). Post-transplant infections can be categorized into two groups: Early and late infections (2). Late infections primarily result from the effects of immunosuppressive medicines, whereas most early infections are attributed to factors unrelated to medication effects (3).
The occurrence of diverse infections in organ transplant recipients poses a persistent challenge, primarily due to the long-term use of immunosuppressive medicines aimed at suppressing immune responses against allograft antigens (4). Among individuals undergoing immunosuppressive treatment, the most common opportunistic infections include cytomegalovirus, herpes simplex, mycobacterium, toxoplasma, Pneumocystis jirovecii, as well as opportunistic fungi such as Candida albicans, other yeasts, Aspergillus, and mucorales (5-7).
Central nervous system (CNS) infections are severe infectious diseases associated with significant complications and mortality rates (8). The specific pathogen involved is influenced by factors such as the age and immune status of the host, as well as the epidemiology of the pathogen. Among the various causes, viral agents are considered common contributors to CNS infections (9). In patients with acute CNS infections, regardless of the underlying cause, the presenting symptoms are often similar and nonspecific. Common nonspecific symptoms include anorexia, fever, headache, irritability, nausea, restlessness, and vomiting (10).
Prompt and accurate diagnosis, followed by timely initiation of appropriate treatment, is crucial in CNS infections. Delays in either diagnosis or treatment can result in increased mortality rates and severe, long-lasting complications for the patient (11).
2. Objectives
Given that there has been no comprehensive study conducted in Iran on this subject, the present study aimed to examine the clinical, laboratory, and radiological features of CNS infections in liver and kidney transplant recipients hospitalized at Montaseriyeh, Imam Reza, and Ghaem hospitals in Mashhad during 2018 - 2022.
3. Methods
This retrospective cross-sectional study involved extracting information from the archives of Montaseriyeh, Imam Reza, and Ghaem hospitals in Mashhad, Iran, regarding patients who underwent liver and kidney transplants between April 2018 and March 2022. Data extraction was performed from the patients’ paper files and the hospital information system (HIS). Among these patients, those confirmed to have CNS infections by their medical team through clinical examination, lumbar puncture, computed tomography (CT) scan, or magnetic resonance imaging (MRI) were included in the study.
Several aspects of the patients’ information were recorded. Demographic information such as age, gender, level of education, occupation, smoking history, and the type of medicines used were documented. Additionally, details regarding the length of hospital stay and, in cases of patient mortality, the cause of death were recorded. Clinical, laboratory, and radiological information related to the specific type of CNS infection were documented. Parameters from the cerebrospinal fluid (CSF) analysis, information about the type of transplant (liver or kidney), immunosuppressive medicines used during the onset of CNS infection symptoms, time since transplantation, prophylactic medicines administered (antiviral, antifungal, antibacterial), therapeutic doses of medications administered, history of transplant rejection, type of organism isolated from the CSF, concurrent blood infection, lung involvement, duration of antimicrobial medicines use, reasons for prescribing antimicrobial medicines, occurrence of other infections during hospitalization, diagnosed type of infection, performed surgeries, neurological symptoms of patients at discharge, and, in cases of patient mortality, the cause of death were all documented.
Common nonspecific symptoms, including fever, headache, nausea and vomiting, anorexia, restlessness, and irritability, as well as specific symptoms such as photophobia, neck stiffness and pain, stupor, coma, seizures, and localized neurological symptoms, were also recorded.
In this study, utmost adherence to ethical considerations was ensured, and the confidentiality of all information recorded in the files was strictly maintained, with no usage other than for this study. In cases where the information in the files was incomplete, efforts were made to gather the relevant information by contacting the patient directly or consulting with the treatment team. This process was carried out after obtaining informed consent from the patient, to minimize any potential breach of confidentiality. Patients with incomplete information who could not be adequately followed up were excluded from the study. It is important to note that these follow-up measures were implemented in a manner that did not lead to any dissatisfaction on the part of the patient’s family.
3.1. Inclusion Criteria
Patients of any age or gender who underwent liver or kidney transplantation between April 2018 and March 2022 and were subsequently diagnosed with CNS infection based on clinical symptoms, CSF analysis, imaging findings (CT/MRI), or microbiological results were included. The diagnosis had to be confirmed by the treating medical team. Only those who provided informed consent (either directly or through the family when applicable) were eligible for inclusion.
3.2. Exclusion Criteria
- Incompleteness of patient file information: Patients were excluded if the necessary clinical symptoms and laboratory findings required for the study were missing from their archived files.
- Non-cooperation of the patient or the patient’s family: If the patient or their family did not cooperate in providing the necessary information to complete the study, the patient was excluded.
3.3. Diagnosis
The CNS infections were diagnosed based on a combination of clinical evaluation, CSF analysis obtained via lumbar puncture, and neuroimaging findings from CT or MRI. In cases where microbial identification was possible, the diagnosis was supported by microbiological testing of CSF or blood. Final confirmation was made by the transplant team based on the integration of clinical presentation, laboratory, radiological, and microbiological data.
3.4. Main Measurable Outcomes
The main measurable outcomes of this study are the clinical, laboratory, and radiological symptoms indicative of CNS infections in liver and kidney transplant patients.
3.5. Sample Size
In this retrospective study, the sample size was determined based on the number of cases recorded in the archives of Montaseriyeh, Imam Reza, and Ghaem hospitals between April 2018 and March 2022. After thorough examinations, a total of 11 patients met the necessary criteria and were included as samples in the study.
3.6. Statistics
The descriptive information, including frequency distribution tables, statistical indicators, and graphs, was analyzed using the statistical analysis software SPSS. The results were analyzed using methods that investigated the relationship between various quantitative variables. To determine the normality of data distribution or to analyze non-parametric data, appropriate tests were employed. These tests aimed to assess the relationship between variables and draw meaningful conclusions from the data. In all calculations, a significance level of 0.05 was considered, indicating the threshold for determining statistical significance.
4. Results
4.1. Characteristics and Outcomes of Transplanted Patients
In the study, a total of 11 cases were included (11 out of 2000 transplanted patients), with 7 cases (63%) being male and 4 cases (37%) being female. Among the 11 cases, 4 male patients (36.4%) received a kidney transplant, 3 male patients (27.3%) received a liver transplant, and 4 female patients (36.4%) received a kidney transplant. Among the male patients, 2 transplants (18.2%) were rejected, and similarly, 2 transplants (18.2%) were rejected among the female patients. Subsequently, 1 case in the male group and 2 cases in the female group underwent re-transplantation. Regarding infection occurrence after transplantation, 2 cases (18.2%) experienced early infections, while 9 cases (81.8%) experienced late infections (after six months) out of the total 11 cases. Furthermore, only 1 patient (9.1%) reported smoking cigarettes (Table 1).
| Variables | Male (n = 7) | Female (n = 4) | Total (n = 11) |
|---|---|---|---|
| Age | 35 ± 17 | 42 ± 19 | - |
| Length of stay | 17 ± 10 | 32 ± 6 | - |
| Transplant type | |||
| Liver | 3 (27.3) | 0 (0.0) | 3 (27.3) |
| Kidney | 4 (36.4) | 4 (36.4) | 8 (72.7) |
| Rejection | 2 (18.2) | 2 (18.2) | 4 (36.4) |
| Retransplant | 1 (9.1) | 2 (18.2) | 3 (27.3) |
| Infection start | |||
| Early | 2(18.2) | 0 | 2 (18.2) |
| Late | 5(45.5) | 4 (36.4) | 9 (81.5) |
| Smoking cigarette | 1 (9.1) | 0 | 1 (9.1) |
a Values are expressed as No. (%) or mean ± SD.
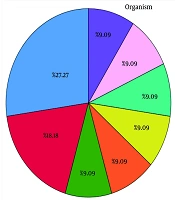
4.2. Types of Organisms Causing Central Nervous System Infections
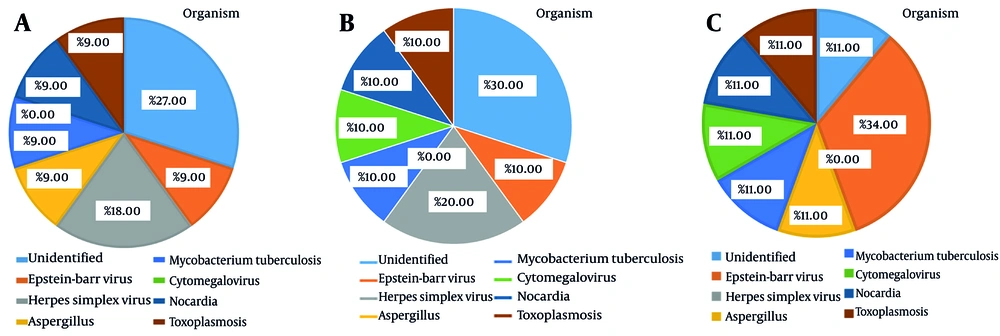
According to Figure 1, the types of organisms causing CNS infections were as follows: Herpes simplex virus at a rate of 18.18%, Aspergillus at a rate of 9.09%, Mycobacterium tuberculosis at a rate of 9.09%, cytomegalovirus at a rate of 9.09%, Nocardia at a rate of 9.09%, toxoplasmosis at a rate of 9.09%, and Epstein-Barr virus at a rate of 9.09%. Additionally, the cause of the infection was unknown in 27.27% of the cases.
4.3. Effect of Medications on the Incidence of Central Nervous System Infections
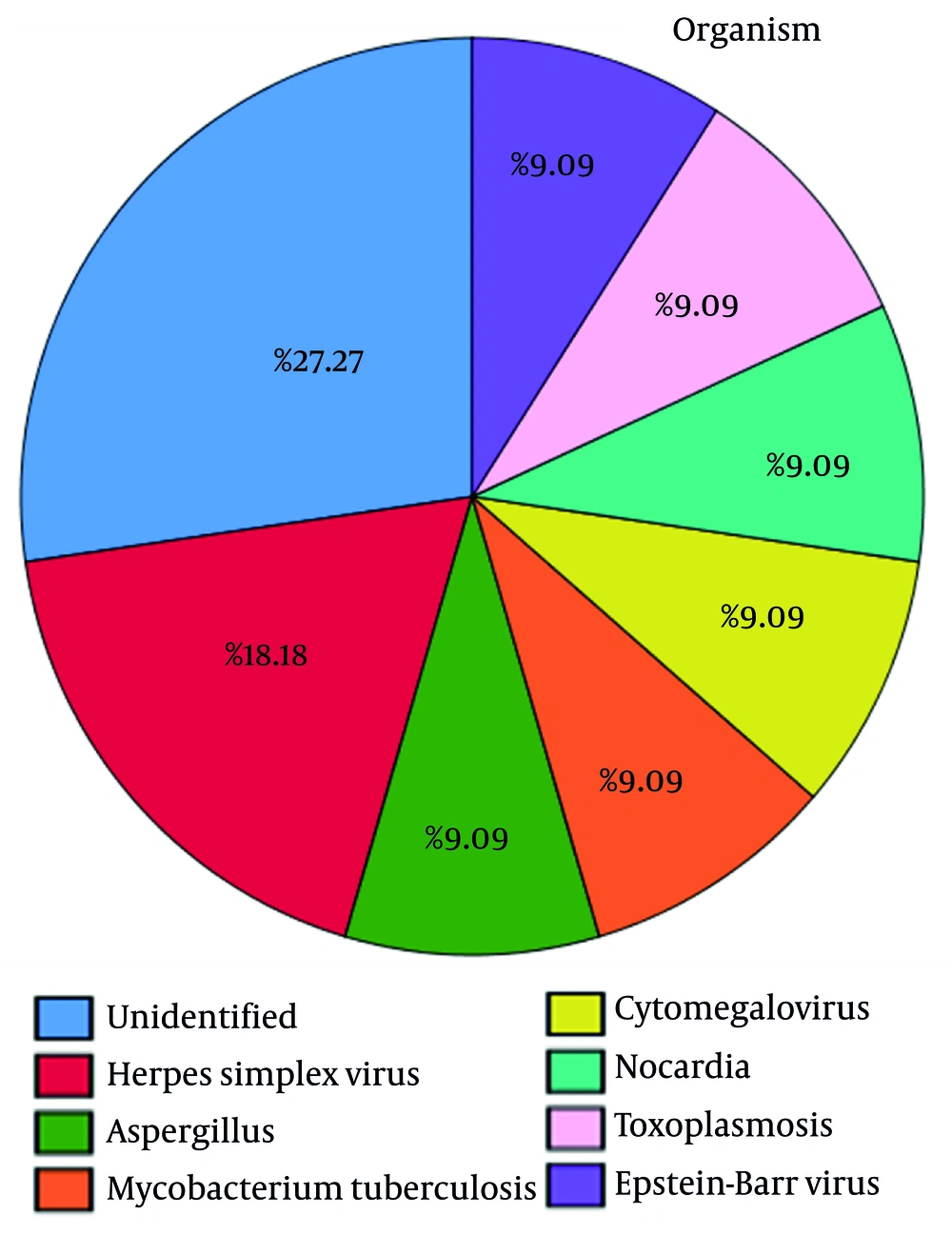
Table 2 reveals that three liver transplant patients used tacrolimus and mycophenolate mofetil, and they had herpes simplex virus, Aspergillus, and M. tuberculosis infections (33% each). According to the findings depicted in Figure 2A, it is evident that the administration of prednisolone resulted in the development of toxoplasmosis (16.67%), as well as infections caused by cytomegalovirus (16.67%) and herpes simplex virus (16.67%). Moreover, it led to an unknown type of infection in 50% of the patients.
| Transplant | Liver (n = 3) | Kidney (n = 8) | ||||||
|---|---|---|---|---|---|---|---|---|
| Immunosuppressant | Tacrolimus | Mycophenolate Mofetil | Prednisolone | Tacrolimus | Azathioprine | Mycophenolate Mofetil | Cyclosporine | Sirolimus |
| Total | 3 (100) | 3 (100) | 6 (75) | 3 (37.5) | 1 (12. 5) | 6 (75) | 5 (62. 5) | 1 (12.5) |
| HSV | 1 (33) | 1 (33) | 1 (12.5) | 1 (12.5) | - | - | - | - |
| Aspergillus | 1 (33) | 1 (33) | - | - | - | - | - | - |
| Mtb | 1 (33) | 1 (33) | - | - | - | - | - | - |
| CMV | - | - | 1 (12.5) | - | - | 1 (12.5) | 1 (12.5) | 1 (12.5) |
| Nocardia | - | - | - | - | 1 (12. 5) | - | 1 (12.5) | - |
| TOXO | - | - | 1 (12.5) | - | - | 1 (12.5) | 1 (12.5) | - |
| EBV | - | - | 1 (12.5) | - | - | - | - | |
| Unidentified | - | - | 3 (37.5) | 1 (12.5) | - | 3 (37.5) | 2 (25) | - |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus; Mtb, mycobacterium tuberculosis; TOXO, toxoplasmosis.
a Values are expressed as No. (%).
Based on the data presented in Figure 2B, it is clear that the administration of tacrolimus medication led to the occurrence of various infections. Specifically, patients demonstrated an increased risk of developing Epstein-Barr virus (16.67%), M. tuberculosis (16.67%), Aspergillus (16.67%), herpes simplex virus (33.33%), and an unidentified infection (16.67%).
According to Figure 2C, the utilization of azathioprine resulted in a 100% incidence of Nocardia infection. Moreover, Figure 2D demonstrates that the administration of mycophenolate mofetil was associated with the development of various infections. Specifically, patients exhibited herpes simplex virus (11.11%), Aspergillus (11.11%), M. tuberculosis (11.11%), cytomegalovirus (11.11%), toxoplasmosis (11.11%), Epstein-Barr virus (11.11%), and an unidentified (33.33%) infection.
Furthermore, cyclosporine administration has been associated with various infections, as depicted in the findings represented in Figure 2E. These infections include cytomegalovirus (20%), Nocardia (20%), toxoplasmosis (20%), as well as an unidentified (40%) infection. Figure 2F showed that the use of sirolimus medication resulted in a 100% incidence of cytomegalovirus infection.
4.4. Association Between Infections, Transplant Rejection, and Re-transplantation
Table 3 provides information on the association between different infections and their effect on transplant rejection and re-transplantation. The findings reveal that among the patients who had infections with herpes simplex virus (33%) and Aspergillus (33%), transplant rejection occurred, while one patient (33%) with herpes simplex virus infection did not experience transplant rejection. Additionally, one patient (33%) with M. tuberculosis infection required re-transplantation. Notably, there were no instances of re-transplantation for patients with herpes simplex virus or Aspergillus infections.
| Transplantation, Status and Organism | HSV | Aspergillus | Mtb | CMV | Nocardia | TOXO | EBV | Unidentified | Total |
|---|---|---|---|---|---|---|---|---|---|
| Liver | |||||||||
| Rejection | |||||||||
| Yes | 1 (33) | 0 | 1 (33) | - | - | - | - | - | 2 (66) |
| No | 1 (33) | - | - | - | - | - | - | - | 1 (33) |
| Re-transplantation | |||||||||
| Yes | - | - | 1 (33) | - | - | - | - | - | 1 (33) |
| No | 1 (33) | 1 (33) | - | - | - | - | - | - | 2 (66) |
| Kidney | |||||||||
| Rejection | |||||||||
| Yes | - | - | - | 1 (12.5) | 1 (12.5) | - | - | - | 2 (25) |
| No | 1 (12.5) | - | - | - | - | 1 (12.5) | 1 (12.5) | 3 (37.5) | 6 (75) |
| Re-transplantation | |||||||||
| Yes | - | - | - | 1 (12.5) | 1 (12.5) | - | - | - | 2 (25) |
| No | 1 (12.5) | - | - | - | - | 1 (12.5) | 1 (12.5) | 3 (37.5) | 6 (75) |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus; Mtb, mycobacterium tuberculosis; TOXO, toxoplasmosis.
a Values are expressed as No. (%).
In the case of kidney transplant recipients, one patient (12.5%) with cytomegalovirus infection and another patient with Nocardia infection experienced rejection. Conversely, none of the patients with herpes simplex virus (12.5%), toxoplasmosis (12.5%), Epstein-Barr virus (12.5%), or unidentified (37.5%) infections faced transplant rejection. However, it is important to mention that among those with cytomegalovirus (12.5%) or Nocardia (12.5%) infections, re-transplantation did occur. Conversely, patients with herpes simplex virus (12.5%), toxoplasmosis (12.5%), Epstein-Barr virus (12.5%), and unidentified (37.5%) infections did not require re-transplantation.
4.5. Clinical Symptoms and Associated Organisms in Central Nervous System Infections
Table 4 displays the symptoms reported by patients and the corresponding organisms responsible for the infections. Among the symptoms reported, fever was the most common, with 10 patients (90.9%) experiencing it. The presence of unidentified organisms accounted for 3 cases (27.3%), while Epstein-Barr virus, herpes simplex virus, Aspergillus, M. tuberculosis, Nocardia, and toxoplasmosis were each associated with 1 case (9.1%) of fever.
| Symptoms | Organisms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unidentified | EBV | HSV | Aspergillus | Mtb | CMV | Nocardia | TOXO | Total | |
| Fever | 3 (27.3) | 1 (9.1) | 2 (18.2) | 1 (9.1) | 1 (9.1) | - | 1 (9.1) | 1 (9.1) | 10 (90.9) |
| Headache | 3 (27.3) | 1 (9.1) | 2 (18.2) | - | 1 (9.1) | 1 (9.1) | 1 (9.1) | 1 (9.1) | 10 (90.9) |
| Nausea | 3 (27.3) | - | 1 (9.1) | 1 (9.1) | 1 (9.1) | 1 (9.1) | 1 (9.1) | 1 (9.1) | 9 (81.8) |
| Vomiting | 2 (18.2) | - | 1 (9.1) | 1 (9.1) | 1 (9.1) | - | 1 (9.1) | 1 (9.1) | 7 (63.6) |
| Loss of appetite | 1 (9.1) | - | 2 (18.2) | - | - | 1 (9.1) | 1 (9.1) | - | 5 (45.5) |
| Irritation | - | - | 2 (18.2) | 1 (9.1) | - | - | - | - | 3 (27.3) |
| Photophobia | - | - | - | - | - | - | - | - | - |
| Neck rigidity | 1 (9.1) | - | - | - | 1 (9.1) | - | - | - | 2 (18.2) |
| Stupor | - | - | 1 (9.1) | - | 1 (9.1) | 1 (9.1) | - | - | 3 (27.3) |
| Seizure | - | 1 (9.1) | 1 (9.1) | - | 1 (9.1) | - | 1 (9.1) | 1 (9.1) | 5 (45.5) |
| Coma | - | - | 1 (9.1) | - | - | - | 1 (9.1) | - | 2 (18.2) |
| Focal neurologic deficit | 2 (18.2) | 1 (9.1) | 1 (9.1) | 1 (9.1) | - | - | 1 (9.1) | - | 6 (54.5) |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus; Mtb, mycobacterium tuberculosis; TOXO, toxoplasmosis.
a Values are expressed as No. (%).
Headache was another frequently reported symptom, observed in 10 patients (90.9%). Similar to fever, unidentified organisms (27.3%), herpes simplex virus (18.2%), Epstein-Barr virus, M. tuberculosis, cytomegalovirus, Nocardia, and toxoplasmosis were associated with 1 case (9.1%) each.
Nausea was experienced by 9 patients (81.8%), with unidentified organisms (27.3%), herpes simplex virus, Aspergillus, M. tuberculosis, cytomegalovirus, Nocardia, and toxoplasmosis each being linked to 1 case (9.1%). Vomiting was reported by 7 patients (63.6%), with unidentified organisms (18.2%), herpes simplex virus, Aspergillus, M. tuberculosis, Nocardia, and toxoplasmosis each associated with 1 case (9.1%).
Loss of appetite was observed in 5 patients (45.5%), with herpes simplex virus (18.2%), unidentified organisms, cytomegalovirus, and Nocardia each being linked to 1 case (9.1%). Seizure was experienced by 5 patients (45.5%). Among the reported cases, Epstein-Barr virus, herpes simplex virus, M. tuberculosis, Nocardia, and toxoplasmosis were each associated with 1 case (9.1%) of seizure.
Additionally, focal neurologic deficit was reported in 6 cases (54.5%). Unidentified organisms (18.2%), Epstein-Barr virus, herpes simplex virus, Aspergillus, and Nocardia were each associated with 1 case (9.1%) of focal neurologic deficit. Other symptoms such as irritation, photophobia, neck rigidity, stupor, and coma were reported less frequently, with different organisms associated with each symptom.
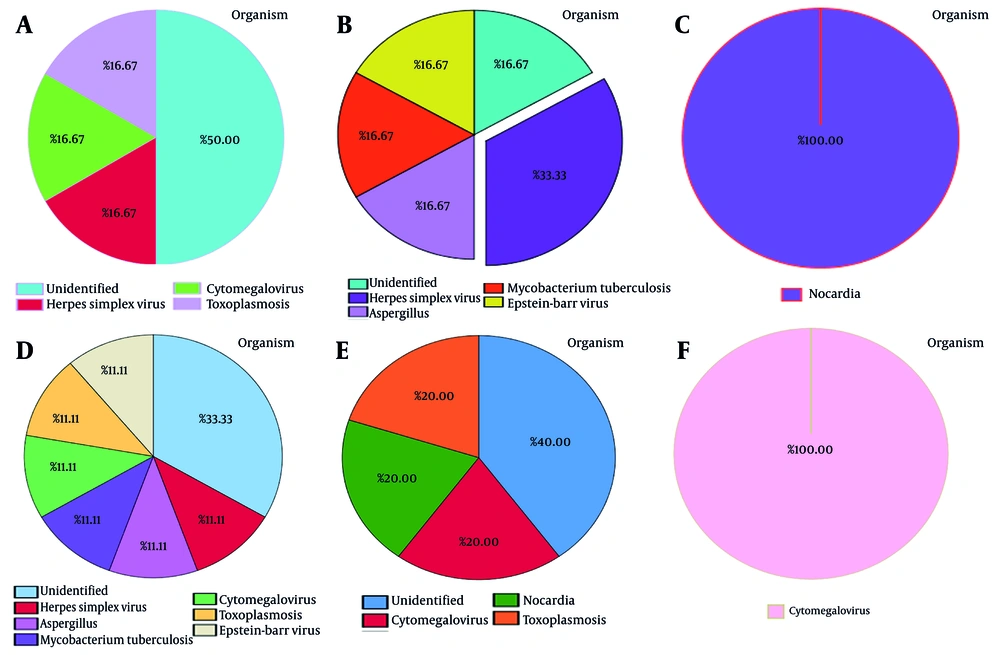
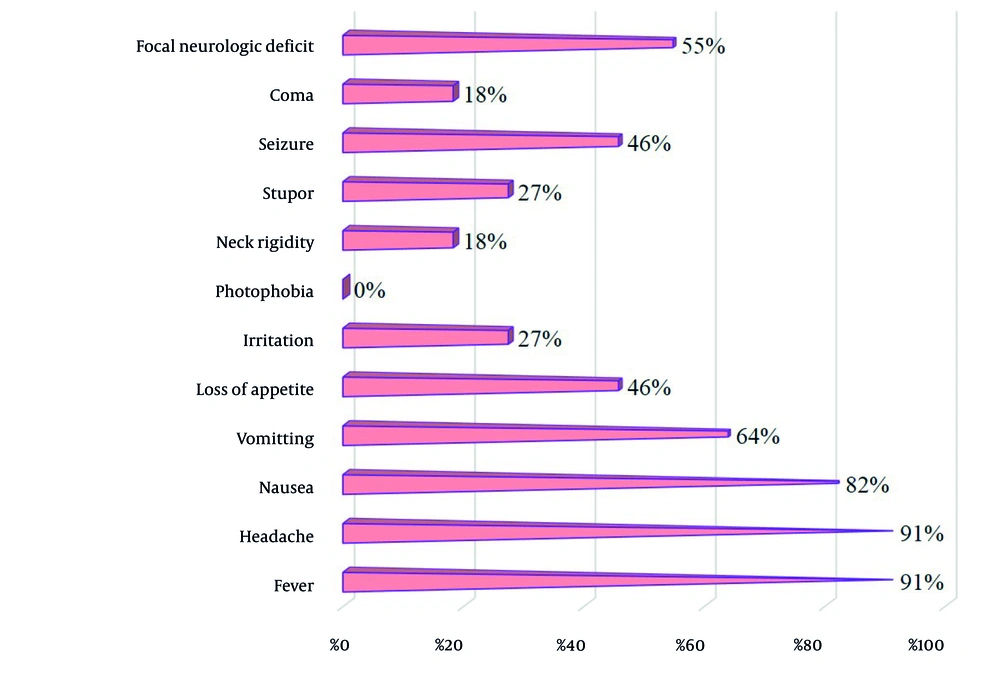
Figure 3 illustrates the symptoms resulting from CNS infections in transplant patients. According to the diagram, the predominant symptoms are fever and headache (91%), followed by nausea (82%) and vomiting (64%). Notably, photophobia is the least commonly reported symptom (0%).
Figure 4A depicts the role of each organism in causing fever. As illustrated in the graph, 27% of cases are caused by unidentified factors. Herpes simplex virus caused 18% of fevers, with Aspergillus, M. tuberculosis, Nocardia, Epstein-Barr virus, and toxoplasmosis accounting for 9% each.
Figure 4B illustrates how each organism contributes to headaches. Thirty percent of the cases had unidentified causes, as indicated in the diagram. Herpes simplex virus was responsible for 20% of headache cases; M. tuberculosis, cytomegalovirus, Nocardia, Epstein-Barr virus, and toxoplasmosis each accounted for 10% of cases. In contrast, none of the reported cases were associated with Aspergillus.
Figure 4C indicates the contribution of each organism to the occurrence of nausea. The graph shows that 34% of the cases had unidentified causes. Among the known pathogens, Aspergillus, M. tuberculosis, Nocardia, toxoplasmosis, cytomegalovirus, and herpes simplex each accounted for an 11% share. Interestingly, Epstein-Barr did not contribute to any of the cases.
4.6. Lung Involvement, Prophylaxis, Discharge, and Mortality Rates in Central Nervous System Infections
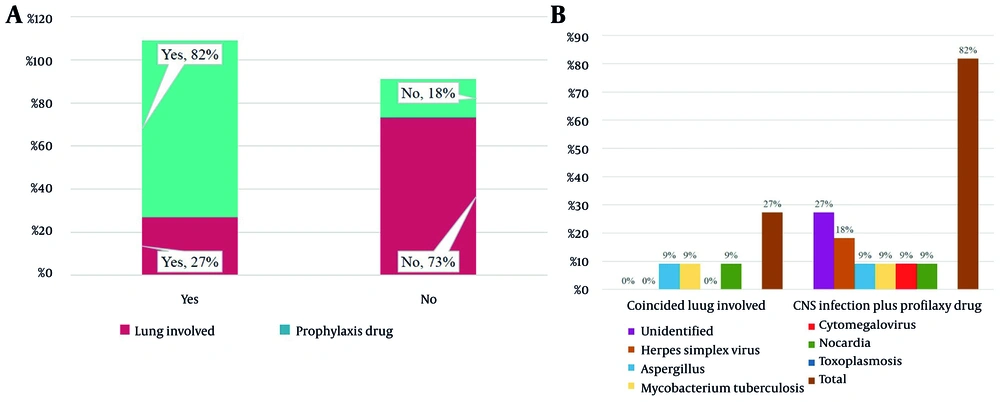
Based on the information provided, it can be observed from Table 5 that out of the 11 people in the sample, 3 patients experienced concurrent lung involvement. The infections causing this lung involvement were identified as Aspergillus, M. tuberculosis, and Nocardia (9.1% each). Among the patients, 2 individuals developed the infection at an early stage (18.2%), while 9 patients (81.8%) experienced it at a later stage. Moreover, it is worth noting that 81.8% of the patients received prophylaxis drugs. Additionally, the table reveals that 7 individuals (64.5%) were discharged from the hospital, while 4 deaths occurred within the sample (36.4%). Among the recorded deaths, herpes simplex virus, M. tuberculosis, and Nocardia were attributed as causes, with one case remaining unknown (9.1% each).
| Organisms | Unidentified | EBV | HSV | Aspergillus | Mtb | CMV | Nocardia | TOXO | Total |
|---|---|---|---|---|---|---|---|---|---|
| Lung involvement | - | - | - | 1 (9.1) | 1 (9.1) | - | 1 (9.1) | - | 3 (27.3) |
| Infection start | |||||||||
| Early | - | - | 1 (9.1) | 1 (9.1) | - | - | - | - | 2 (18.2) |
| Late | 3 (27.3) | 1 (9.1) | 1 (9.1) | - | 1 (9.1) | 1 (9.1) | 1 (9.1) | 1 (9.1) | 9 (81.8) |
| Prophylaxis drug | 2 (18.2) | 1 (9.1) | 2 (18.2) | 1 (9.1) | 1 (9.1) | 1 (9.1) | 1 (9.1) | - | 9 (81.8) |
| Discharge status | |||||||||
| Discharge | 2 (18.2) | 1 (9.1) | 1 (9.1) | 1 (9.1) | - | 1 (9.1) | - | 1 (9.1) | 7 (64.5) |
| Death | 1 (9.1) | - | 1 (9.1) | - | 1 (9.1) | - | 1 (9.1) | - | 4 (36.4) |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus; Mtb, mycobacterium tuberculosis; TOXO, toxoplasmosis.
a Values are expressed as No. (%).
4.7. Timing of Symptom Onset in Central Nervous System Infections
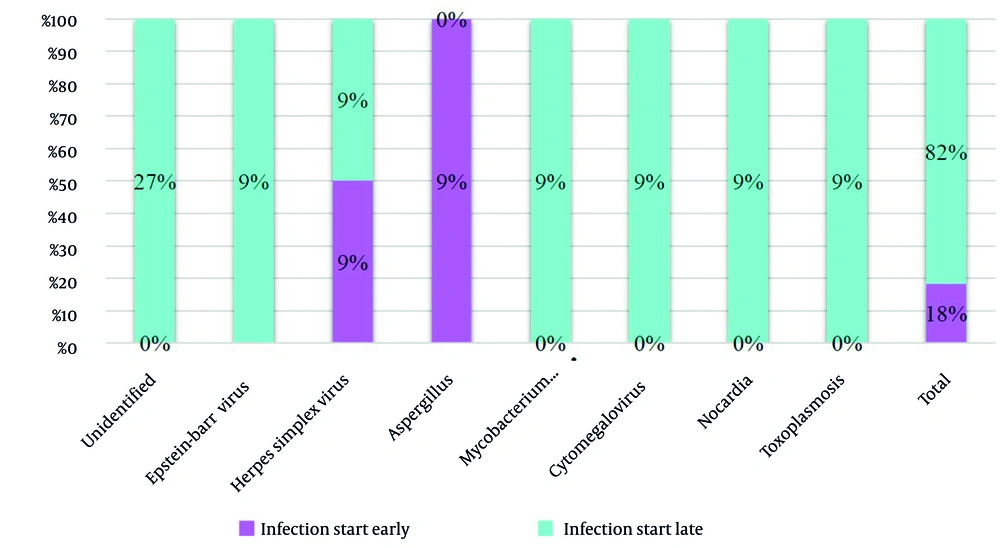
Figure 5 elucidates that the symptoms of herpes simplex virus and Aspergillus infection manifested early (18%), while in other cases, symptom onset occurred at a later stage (82%).
4.8. Effect of Prophylactic Medication on Concurrent Lung Involvement in Central Nervous System Infections
The information presented in Figure 6A illustrates the relationship between the occurrence of simultaneous lung involvement and the utilization of a prophylactic medication regimen. Based on the data, it can be inferred that patients who received the prophylactic medication regimen had a lower incidence of lung involvement compared to those who did not receive such medication. In patients who received prophylactic drugs, the rates of CNS infections were as follows: Herpes simplex virus (18%), Epstein-Barr virus, Aspergillus, M. tuberculosis, cytomegalovirus, Nocardia, and toxoplasmosis (9% each). Additionally, the incidence of pulmonary involvement coincided with the following pathogens: Aspergillus, M. tuberculosis, and Nocardia (9% each) (Figure 6B).
4.9. Spinal Fluid Collection, Patient Outcomes, Computed Tomography/Magnetic Resonance Imaging Findings, and Reasons for Transplant in Central Nervous System Infections
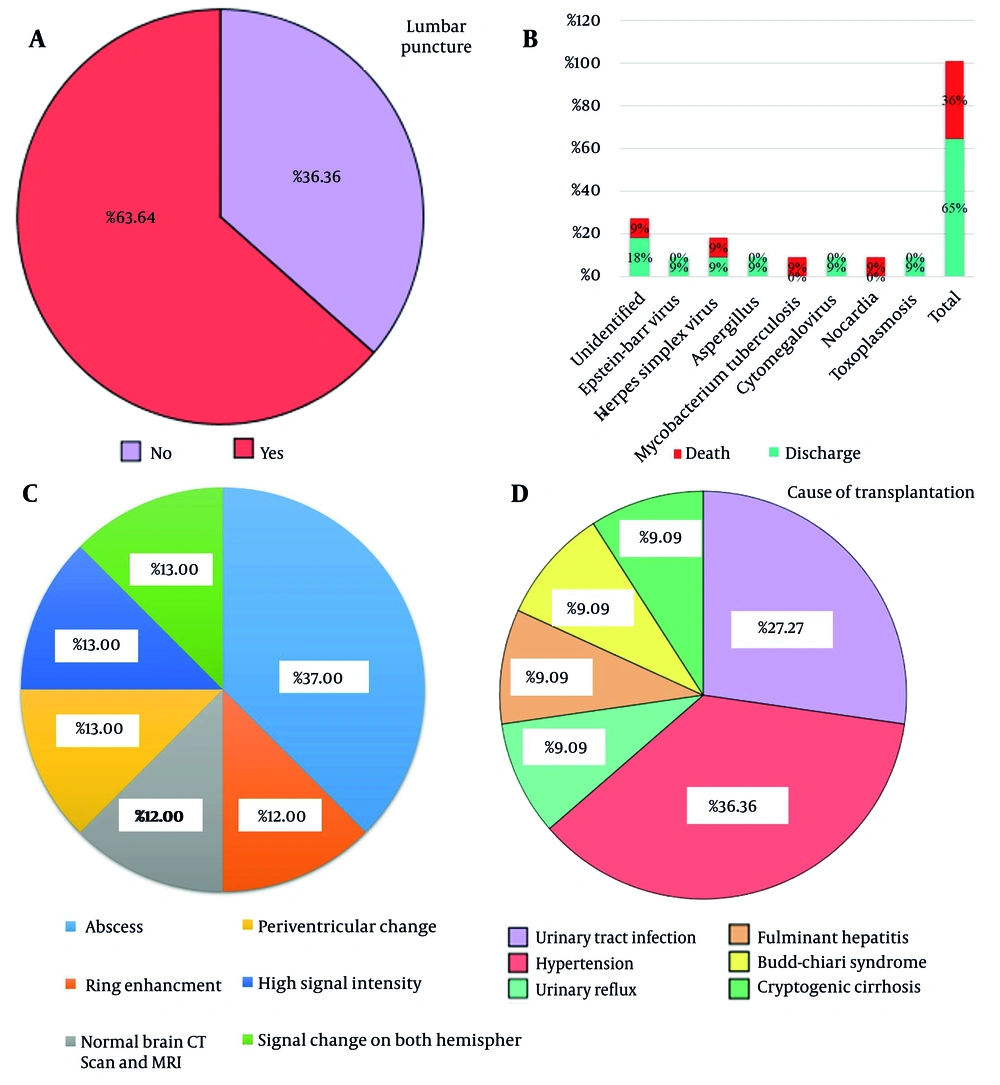
The data presented in Figure 7A demonstrates that spinal fluid was collected from patients in 63.64% of cases, enabling the identification of the cause of infection in these individuals. However, in 36.36% of cases, spinal fluid was not obtained, resulting in the cause of the infection remaining unidentified.
Figure 7B shows the status of transplanted patients. The results showed that 4 patients, equivalent to 36%, ultimately died. Among the causes of death, unidentified factors, herpes simplex virus, M. tuberculosis, and Nocardia each accounted for 9% of the cases. Confidently, 64% recovered and were discharged.
Table 6 and Figure 7C present a summary of cases along with their corresponding CT/MRI findings and radiological manifestations. The CT/MRI scans revealed various results, including normal brain CT scan and MRI (12%), abscesses (37%), ring enhancement (12%), periventricular changes (13%), high signal intensity (13%), and signal changes (13%). Radiological manifestations included lung nodules, lacunar infarction, sphenoid thickening, and brain edema.
| Case Number | CT/MRI | Radio Manifestation |
|---|---|---|
| 1 | 1 | Normal |
| 2 | 1 abscess (hypo density) | Lung nodule |
| 3 | 1 ring enhancement | 1 |
| 4 | 0 | 0 |
| 5 | 1 brain CT and MRI NL | - |
| 6 | 0 | 0 |
| 7 | 1 periventricular change (nonspecific) lacunar infarction | Sphenoid thickening |
| 8 | 1 multiple abscesses on MRI | Lung centrilobular nodule on CT scan |
| 9 | 1 brain abscess 20 × 19 mm | - |
| 10 | 1 high signal intensity on parenchyma and periventricular and sub cortical bulging and vasculitis and mastoiditis | Brain edema and maxillary sinusitis on CT scan |
| 11 | 1 signal change on both hemisphere | - |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
In Figure 7D, the reasons for transplant in patients are presented. The data reveals that urinary tract infection accounted for 27.27% of the cases, while hypertension was the reason for transplantation in 36.36% of patients. Urinary reflux, fulminant hepatitis, Budd-Chiari syndrome, and cryptogenic cirrhosis each constituted 9.09% of the cases.
5. Discussion
The study examined CNS infections in transplant patients and investigated various aspects such as lung involvement, prophylaxis, discharge rates, mortality rates, timing of symptom onset, spinal fluid collection, patient outcomes, CT/MRI findings, and reasons for transplant. The data disclosed that out of the total 11 cases, 7 (63%) were male and 4 (37%) were female. Among the male patients, 4 (36.4%) received a kidney transplant, 3 (27.3%) received a liver transplant, and among the female patients, 4 (36.4%) received a kidney transplant. In the male group, 2 transplants (18.2%) were rejected, as well as 2 transplants (18.2%) in the female group. Subsequently, one case in the male group and two cases in the female group underwent re-transplantation. After transplantation, a total of 2 cases (18%) experienced early infections, while 9 cases (82%) experienced late infections.
The identified organisms causing CNS infections were as follows: Herpes simplex virus (18.18%), Aspergillus (9.09%), M. tuberculosis (9.09%), cytomegalovirus (9.09%), Nocardia (9.09%), toxoplasmosis (9.09%), Epstein-Barr virus (9.09%), as well as unidentified causes in 27.27% of cases. Prednisolone was administered in 4 groups (toxoplasmosis, unidentified, cytomegalovirus, and herpes simplex virus). Tacrolimus was used in patients with Epstein-Barr virus, M. tuberculosis, Aspergillus, herpes simplex virus, and unidentified infections. Azathioprine was only used in the Nocardia group. Mycophenolate mofetil was administered to all patients. Cyclosporine was used in the cytomegalovirus, Nocardia, toxoplasmosis, and unidentified groups. Sirolimus was only used in cytomegalovirus patients.
The findings shed light on several important aspects of CNS infections in this specific patient population. The incidence of CNS infections in solid organ transplant recipients varies greatly between studies, ranging from 1% to 24%. These rates are influenced by parameters such as the study period and the transplanted organ (12-16). In our study, CNS infections occurred in 0.55% of transplanted patients, which is consistent with recent data showing 0.8% of CNS infections in solid organ transplants in Switzerland (13). However, our analysis found a distinct spectrum of infections compared to previous solid organ transplant recipient groups. This disparity can be related to geographical variations in epidemiology and differences in preventive strategies employed (13, 17).
Our study included cases of cerebral toxoplasmosis, reflecting the epidemiology of these infections in the general population of Mashhad, Iran. The timing of CNS infections following transplantation varies considerably depending on the etiology. Aspergillus infection is most commonly seen as an early post-transplant event, consistent with previous studies (13, 18-20). In contrast, infections with Epstein-Barr virus, M. tuberculosis, cytomegalovirus, Nocardia, and toxoplasmosis have a delayed onset after transplantation. Other investigations have also indicated that nocardiosis has a delayed onset after transplantation (13, 21).
In the present study, concurrent pulmonary involvement was observed in patients who did not receive prophylaxis drugs. Another study also reported that concomitant pulmonary involvement has been observed in over 70% of patients diagnosed with CNS aspergillosis, indicating the potential hematogenous spread of the infection (13). Our findings emphasize the challenge in diagnosing CNS infections in liver and kidney transplant recipients due to the atypical or suppressed clinical presentation and the frequent presence of concurrent non-infectious complications. As a result, doctors should set a low threshold for recognizing infection as a possible differential diagnosis in SOT recipients who exhibit modest and nonspecific neurological symptoms.
It is important to recognize the various limitations of this study. First, it was not possible to perform a comprehensive analysis of the risk factors linked to the development of CNS infections because of the infrequency and heterogeneity of these infections in recipients of liver and kidney transplants. The involvement of multinational cohorts would be required to address this. It is also critical to remember that the infection epidemiology found in our study only accounts for the situation in Mashhad, Iran. Larger multinational cohorts would be essential to have a deeper understanding of the burden of diseases in various geographic contexts. Nonetheless, the study’s extensive inclusion of almost all liver and kidney transplant recipients, as well as the systematic follow-up procedure, ensures that the data presented accurately reflect the epidemiology and burden of CNS infections in the modern transplant era, particularly in a Middle Eastern country. Additionally, no subgroup analysis — such as comparisons between liver and kidney transplant recipients — was performed, as the small sample size (n = 11) limited the statistical power to conduct meaningful comparisons. Future studies with larger cohorts are necessary to explore potential differences in infection patterns and outcomes across transplant types.
5.1. Conclusions
In conclusion, compared to previous research, there has been a decline in the incidence of CNS infections in the modern era marked by immunosuppression and antimicrobial prophylaxis. Nevertheless, CNS infections — bacterial and viral infections in particular — remain a major cause of mortality for recipients of liver and kidney transplants. The management of CNS infections in liver and kidney transplant recipients is still a challenging task, despite advances in diagnostic methods and therapeutic strategies. This highlights the need for increased awareness and the application of proactive diagnostic and therapeutic strategies in this patient population.