1. Background
Tuberculosis remains a severe threat to the lives of liver transplant patients due to high fatality rates (1, 2). In this vulnerable population, tuberculosis infection is most commonly caused by the reactivation of latent infection, but it can also be transmitted from an infected organ after donation (2). However, diagnosing and treating tuberculosis in the post-transplant period involves numerous complications. Delays in diagnosis are prevalent due to unusual clinical symptoms and an elevated incidence of extra-pulmonary tuberculosis (about 60%) among solid organ recipients. Furthermore, the lack of precise diagnostic tools complicates the diagnosis procedure. The coexistence of various medical conditions raises concerns about possible drug-drug interactions between anti-tuberculosis medication and cyclosporine, a routinely used immunosuppressive medicine in liver transplant recipients. Additionally, the risk of hepatotoxicity with anti-tuberculosis medications adds another degree of difficulty (3). Alarming statistics show that the death rate linked with tuberculosis infection in transplant patients is around 29%, highlighting its role as one of the most life-threatening infectious diseases in this population (4).
Immunosuppression is an established risk factor for tuberculosis recurrence, particularly in the context of organ transplantation. Tuberculosis infection occurs in approximately 1.2 - 6.4% of transplant cases worldwide, with frequency reaching 15% in tuberculosis-endemic countries (5). Reactivation of latent tuberculosis infection (LTBI) is the most common type of tuberculosis presentation after transplantation (2). In the general population, 5 - 10% of people with healthy immune systems who have been exposed to Mycobacterium tuberculosis will develop active tuberculosis at some point throughout their lives. However, this risk increases dramatically, with estimates ranging from 10 to 70 times greater in persons undertaking immunosuppressive regimens, such as transplant recipients (2, 6).
Prophylactic use of isoniazid is recommended for high-risk populations (7). Isoniazid is an antibiotic prescribed for the treatment and prevention of tuberculosis. It is frequently used in combination with other medications to prevent the development of drug-resistant tuberculosis (8). This medicine suppresses the growth of bacteria and is only effective against infections caused by bacteria, not viral diseases such as the common cold or the flu. Isoniazid attacks M. tuberculosis by inhibiting the formation of mycolic acid, a key component of the bacterial cell wall (9).
2. Objectives
Therefore, the present study aimed to investigate the treatment, management, and prognosis of LTBI in liver transplant recipients at Montaseriyeh Hospital in Mashhad, during the years 2013 - 2021. This was achieved by conducting a comprehensive review of the medical records of 450 liver transplant patients, which provided valuable insights into the donor-recipient purified protein derivative (PPD) and interferon-gamma release assay (IGRA) results, details of prophylaxis drug type and dosage, duration of previous LTBI treatment, as well as the patient’s prognosis during the 36-month follow-up period after transplantation. By examining these factors, the study aimed to contribute to the development of effective strategies for the prevention and management of tuberculosis in liver transplant recipients, ultimately improving patient outcomes and reducing the incidence of tuberculosis-related complications and mortality.
3. Methods
3.1. Study Approval and Data Extraction
The study was approved by the Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.MEDICAL.REC.1400.639). Data for this retrospective cross-sectional study were extracted from the archives of Montaseriyeh Hospital, Mashhad. The information referred to liver transplant recipients between the years 2013 - 2021. The data extraction involved a review of patients’ paper files and accessing the hospital information system (HIS). Initially, all files of liver transplant recipients during the 8 years were reviewed. Patients who exhibited a positive PPD IGRA response in either the donor or recipient were included in the study. In cases where the information in the files was incomplete, efforts were made to supplement the missing details by directly contacting the patients or conducting personal visits. Patients were assured of the confidentiality of their information, which would be solely used for the study.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria for the study comprised patients who received liver transplants and had positive PPD IGRA results for either the donor or recipient. Exclusion criteria were applied to cases where hospital records were inaccurately completed or for patients who did not receive isoniazid treatment due to allergies or other reasons.
3.3. Data Collection
The data collection process began by recording the demographic information of each patient, including age, gender, occupation, smoking status, and length of hospital stay. Clinical and laboratory information was subsequently documented, which included the history of specific medications, presence of underlying diseases, prior history of tuberculosis, dosage and duration of isoniazid treatment, the occurrence of tuberculosis or other infections post-transplantation, diagnosed infections, performed surgeries, transplant rejection, cause of patient death (if applicable), and laboratory results such as tuberculin skin test (TST) outcomes and liver enzyme levels to assess liver toxicity.
3.4. Main Measurable Outcomes
The main outcome of the study was to determine the decision regarding the administration of tuberculosis latent infection prophylaxis (TBLLIBI) based on the PPD IGRA response of the donor or transplant recipient. Additionally, the study aimed to assess the dose and duration of isoniazid use as prophylaxis, evaluate liver toxicity, and examine transplant rejection after receiving isoniazid in liver transplant recipients.
3.5. Tuberculin Skin Test Evaluation
The results of the TST were assessed by trained infectious disease specialists at Montaseriyeh Hospital. All readings were performed 48 to 72 hours after intradermal injection of PPD. The diameter of induration (not erythema) was measured in millimeters using a transparent ruler, following standard guidelines. Only measurements showing an induration equal to or greater than the defined threshold for immunocompromised patients were considered positive.
3.6. Sample Size and Statistical Method
Owing to the restricted patient pool, a census sample strategy was employed, drawing from the patient files of liver recipient patients registered from 2013 to 2021. Thirty individuals were gathered for analysis.
3.7. Statistical Analysis
The data was described using appropriate statistical tables and indices such as the mean, standard deviation, and quartiles. The dispersion of qualitative variables was described using a bar graph, whereas the dispersion of quantitative variables was presented using a histogram. To begin, the Shapiro-Wilk test and the Q-Q plot diagram were used to check the normal distribution of the independent variables of this study at different intervals, i.e., the length of isoniazid use and the level of liver enzymes in one-month intervals over the first four months. Due to the non-normal distribution of data in the independent variables, the Friedman test was used to compare the average of liver enzymes at different times. In addition, a logistic regression statistical test was utilized to explore the semantic link between the level of liver enzymes in each period and the duration of isoniazid use with mortality rates. Python 3 was used for data analysis, and the significance level for all tests was less than 5%.
4. Results
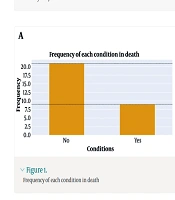
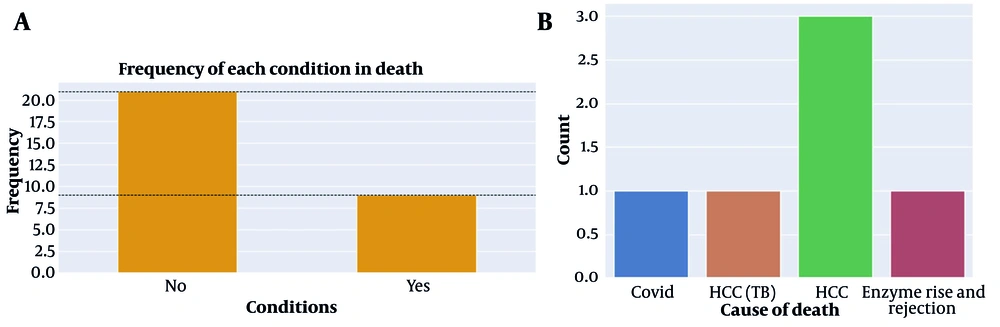
The target group for this study consisted of liver transplant patients at Montaseriyeh Hospital in Mashhad during the period of 2013 - 2021. The study involved the examination of over 400 transplant patients, focusing on determining the criteria for patients to receive isoniazid. In this particular population, patients who had either a positive PPD or IGRA test in either the recipient or transplant donor were prescribed isoniazid as a prophylactic measure for tuberculosis or LTBI. Among the patients under investigation, a total of 30 individuals met the eligibility criteria and had received isoniazid. Data about these patients were extracted for further analysis. Specifically, the collected data included the duration of isoniazid usage, which extended up to 9 months at the end of the prophylaxis period. Additionally, the levels of liver enzymes [serum glutamic oxaloacetic transaminase (SGOT) and serum glutamate pyruvate transaminase (SGPT)] were recorded during the first 4 months of treatment. Furthermore, the outcomes of these patients, in terms of survival or death, were documented. In the event of patient mortality, the cause of death was also collected as part of the data (Table 1 and Figure 1).
| Variables | Coefficient | SE | Z | P-Value | CI (2.5%) | CI (97.5%) |
|---|---|---|---|---|---|---|
| INH duration | -2.1 | 1.3 | -1.6 | 0.11 | -4.7 | 0.5 |
| SGOT1 | -2.8 | 1.2 | -2.3 | 0.021 | -5.2 | -0.4 |
| SGPT1 | -3.4 | 1.4 | -2.5 | 0.012 | -6.1 | -0.7 |
| SGOT2 | -0.9 | 0.9 | -1.1 | 0.254 | -2.6 | 0.7 |
| SGPT2 | -1.2 | 0.7 | -1.7 | 0.097 | -2.5 | 0.2 |
| SGOT3 | -1.7 | 1.2 | -1.5 | 0.14 | -4.0 | 0.6 |
| SGPT3 | -1.6 | 0.9 | -1.7 | 0.089 | -3.5 | 0.2 |
| SGOT4 | -0.5 | 1.2 | -0.4 | 0.69 | -2.9 | 1.9 |
| SGPT4 | -0.8 | 1.2 | -0.6 | 0.518 | -3.2 | 1.6 |
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamate pyruvate transaminase.
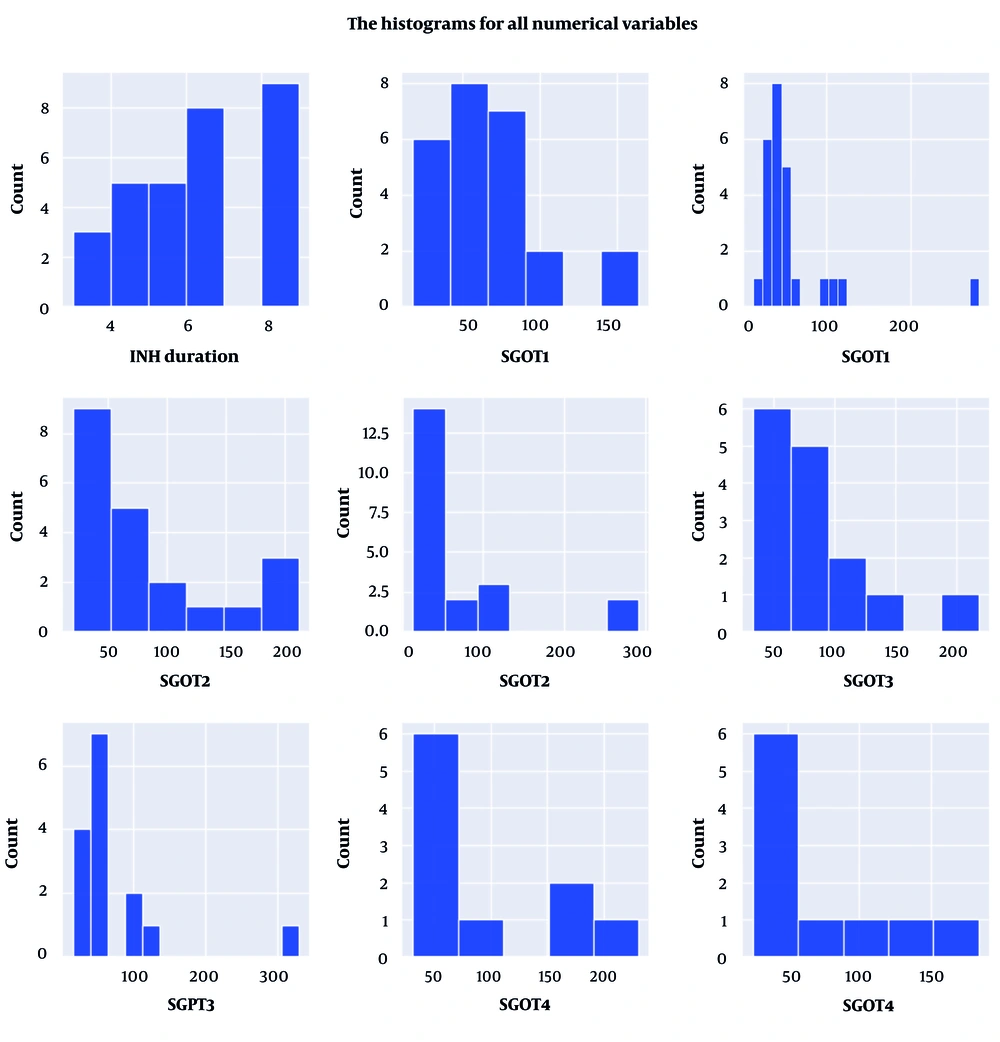
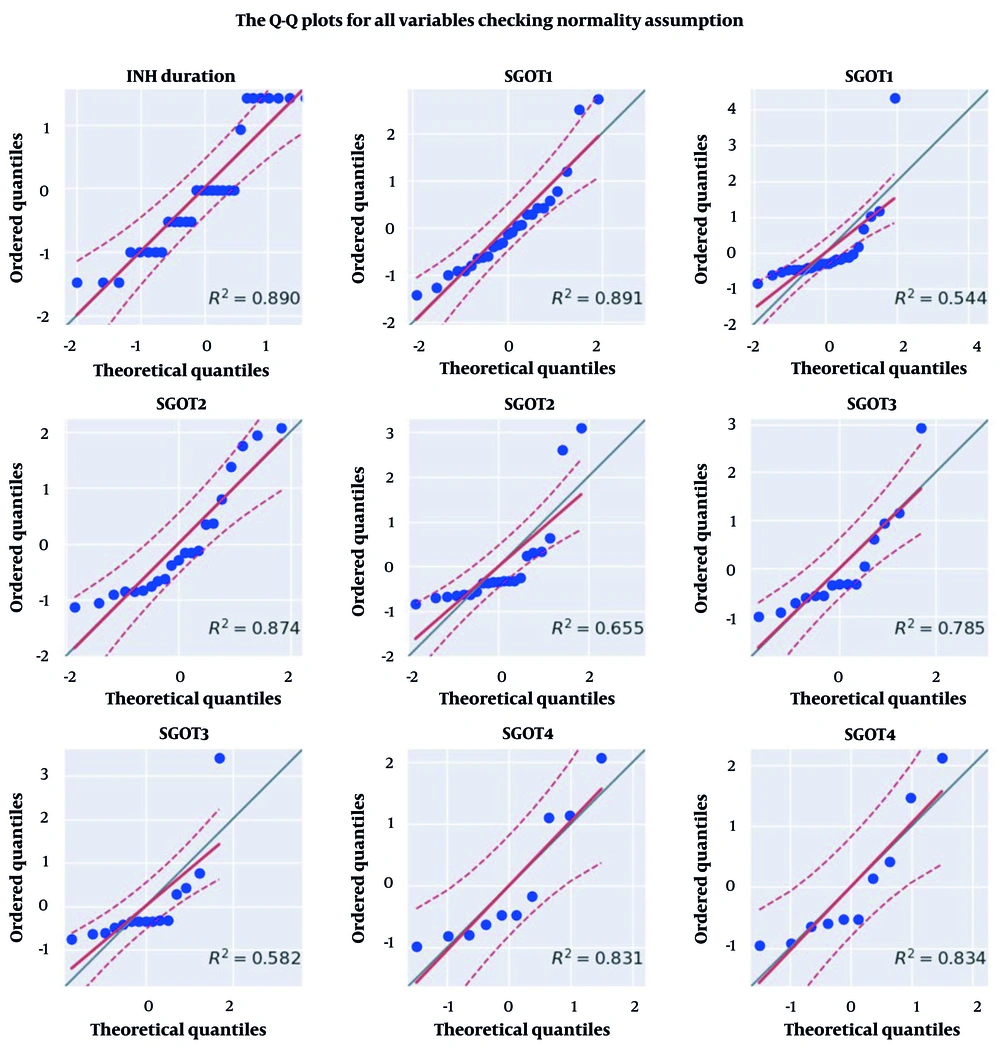
Subsequently, the collected information was subjected to analysis using appropriate methods. The description and histogram depicting the distribution of isoniazid use duration and liver enzyme levels in the studied subjects are presented in Table 2 and Figure 2, respectively. Based on the analysis (Table 3 and Figure 3), it was observed that the data distribution for both the duration of isoniazid use and the level of liver enzymes in the studied subjects did not meet the assumption of normality. None of these variables exhibited a normal distribution at a significance level of 0.05, based on the Q-Q plot. Consequently, for future analyses, non-parametric tests, such as Friedman’s test, should be employed to compare the average levels of liver enzymes across different intervals. Hence, Friedman’s test was employed to compare the average levels of liver enzymes among the study subjects across different intervals. The SGOT (P = 0.87) and SGPT (P = 0.78) levels did not show a significant increase when compared to each other across different intervals (P > 0.05).
| Variables | INH Duration | SGOT1 | SGPT1 | SGOT2 | SGPT2 | SGOT3 | SGPT3 | SGOT4 | SGPT4 |
|---|---|---|---|---|---|---|---|---|---|
| Count | 30.0 | 25.0 | 25.0 | 21.0 | 21.0 | 15.0 | 15.0 | 10.0 | 10.0 |
| Mean | 6.1 | 67.7 | 57.2 | 87.4 | 72.3 | 80.2 | 73.2 | 95.4 | 74.7 |
| Std | 2.1 | 35.9 | 52.8 | 61.4 | 72.5 | 48.8 | 77.3 | 68.8 | 53.6 |
| Min | 3.0 | 18.0 | 12.0 | 20.0 | 13.0 | 33.0 | 17.0 | 31.0 | 26.0 |
| 25% | 4.2 | 45.0 | 33.0 | 38.0 | 29.0 | 52.5 | 39.5 | 45.7 | 42.5 |
| 50% | 6.0 | 63.0 | 42.0 | 71.0 | 49.0 | 65.0 | 48.0 | 65.0 | 48.0 |
| 75% | 8.7 | 83.0 | 50.0 | 110.0 | 89.0 | 95.5 | 71.5 | 147.2 | 92.5 |
| Max | 9.0 | 164.0 | 281.0 | 212.0 | 292.0 | 218.0 | 329.0 | 231.0 | 183.0 |
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamate pyruvate transaminase.
| Variables | W | P-Value | Normal |
|---|---|---|---|
| INH duration | 0.87 | 0.001 | False |
| SGOT1 | 0.89 | 0.014 | False |
| SGPT1 | 0.57 | < 0.001 | False |
| SGOT2 | 0.86 | 0.006 | False |
| SGPT2 | 0.67 | < 0.001 | False |
| SGOT3 | 0.79 | 0.003 | False |
| SGPT3 | 0.61 | < 0.001 | False |
| SGOT4 | 0.82 | 0.028 | False |
| SGPT4 | 0.83 | 0.031 | False |
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamate pyruvate transaminase.
When examining each variable separately in relation to the mortality status of the patients, it was found that the levels of liver enzymes SGOT and SGPT at the end of the first month after isoniazid consumption had a significant association with patient survival (SGOT1 P = 0.02, SGPT1 P = 0.01). Specifically, higher levels of these liver enzymes at the end of the first month after starting isoniazid consumption were found to be significantly associated with an increased risk of mortality in patients. However, the duration of isoniazid use and the levels of liver enzymes at the end of subsequent months, excluding the first month, did not show a significant relationship with the survival status of the patients (P > 0.05).
5. Discussion
This retrospective cross-sectional study aimed to investigate the management of LTBI in liver transplant recipients and evaluate prognostic factors related to the administration of isoniazid prophylaxis and patient outcomes. Our analysis included data from 30 liver transplant recipients who met the eligibility criteria and received isoniazid prophylaxis. The duration of isoniazid usage ranged up to 9 months. Our data showed that the distribution of isoniazid use duration and liver enzyme levels did not follow a normal distribution. Interestingly, we observed no significant increase in liver enzyme levels (SGOT and SGPT) across different intervals. However, when examining each variable separately, higher SGOT and SGPT levels at the end of the first month after isoniazid consumption were significantly associated with increased mortality risk. The duration of isoniazid use and liver enzyme levels in subsequent months did not exhibit a significant relationship with patient survival.
Iran, an endemic country for tuberculosis, has a high frequency of tuberculosis infection, with 14 cases per 100,000 people (2). This situation presents a substantial difficulty for people with impaired immune systems, especially those who have had organ transplants. The incidence of tuberculosis among organ recipients is significantly higher, ranging from 20 to 74 times that of the general population. Furthermore, individuals who had immunosuppressive treatment before transplantation have a six-fold higher chance of acquiring tuberculosis following the surgery (2, 10). These findings highlight the significance of comprehensive tuberculosis screening before transplantation. Screening for LTBI before transplantation presents challenges, with variations in approaches among different centers. The use of the IGRA test adds complexity to the screening process, and its effectiveness on the outcomes of liver transplant recipients remains unclear. In a retrospective study, the positive rate of the QuantiFERON-Tuberculosis Gold test (QFT) before liver transplant was found to be 13.5%, while none of the positive cases progressed to active tuberculosis (10).
Despite the complexity associated with the diagnosis and treatment of latent tuberculosis, most transplant centers, including our center, perform latent tuberculosis screening and provide treatment when necessary. In a previous study in Tehran, Iran, the positive TST results in cirrhotic patients on the waiting list were 15.9% (11), which is lower than the reported rates of positive TST among liver recipients in Canada (24.2%) (12) and Italy (44%) (13). However, in the present study, the rate of LTBI was found to be 7.5%. Latent tuberculosis treatment is essential, particularly for high-risk individuals such as solid organ transplant recipients (2). Following the transplant surgery, there was a notable decrease in the number of active tuberculosis cases since all patients in our research who tested positive for the PPD test were given prophylactic treatment. After receiving a transplant, 9 of the latent tuberculosis patients experienced active tuberculosis. Before receiving an organ transplant, latent tuberculosis patients are advised to take isoniazid once a day for nine months.
On the other hand, reports of the chemical toxicity of isoniazid are not entirely consistent (8). In transplant patients without a history of liver disease, the risk of hepatotoxicity related to isoniazid prophylaxis appears to be low, and in certain candidates for liver transplantation who have compensated hepatic disease, it may even be well tolerated (2, 14). However, it is crucial to emphasize the importance of careful monitoring for adverse effects and the potential elevation of liver enzymes during the administration of isoniazid (14). According to a study by Moon et al., due to the risk of isoniazid-induced hepatotoxicity, it is advisable to avoid using isoniazid in the early post-liver transplant period. Instead, it is recommended to wait until the recipient’s liver function has stabilized before initiating isoniazid prophylaxis in liver transplant recipients (15).
Isoniazid was successfully used in our trial to treat latent tuberculosis before liver transplantation, and no side effects were noted. Surprisingly, we found no significant rise in liver enzyme levels, particularly serum SGOT and SGPT, across different intervals. This shows that isoniazid prophylaxis may not have any significant effect on liver function in liver transplant recipients with latent tuberculosis. However, when each variable was examined separately, greater SGOT and SGPT levels at the end of the first month after isoniazid consumption were substantially related to an increased mortality risk. These findings highlight the significance of monitoring liver enzyme levels throughout the early phases of isoniazid treatment in order to identify patients at increased risk of serious side effects. The lack of a significant association between the duration of isoniazid use and liver enzyme levels in the following months showed that prolonged isoniazid treatment may have little influence on liver function in this patient population. However, it is crucial to consider individual patient characteristics and closely monitor liver enzyme levels throughout the treatment period to ensure patient safety and optimize outcomes.
5.1. Conclusions
Finally, our study focused on the management of LTBI in liver transplant recipients. The study found that the duration of isoniazid use varied among individuals, although there was no significant rise in liver enzyme levels (SGOT and SGPT) during the treatment period. Higher SGOT and SGPT levels at the end of the first month of isoniazid consumption were substantially related to an increased risk of mortality. These findings highlight the necessity of early monitoring of liver enzyme levels to identify patients who are at a higher risk of adverse consequences. More study is needed to improve the management of LTBI in liver transplant recipients and provide better patient care in this vulnerable population.
5.2. Strengths and Limitations
The inclusion of a homogeneous sample of liver transplant recipients with LTBI and the thorough examination of numerous prognostic markers, such as the length of isoniazid use and liver enzyme levels, are two of the strongest points of our study. There are a few limitations, though, that must be noted. First, the retrospective design of the study introduces biases by design and makes it more difficult to demonstrate causation. Secondly, the limited sample size could potentially impact the applicability of our results. To validate our findings, larger sample sizes and prospective designs should be used in future research.