1. Introduction
According to the World Health Organization (WHO), almost 1.4 million cases of hepatitis A are reported each year worldwide (1), while the hepatitis A virus (HAV) infection rate is predicted to be ten times higher. In the Russian Federation, the incidence of hepatitis A since 2021 ranged from 1.5 to 2.4 per 100,000; in 2023, 3,545 patients with HAV were registered. Hepatitis A is one of the most common infectious diseases, generally benign, and extremely rarely, in less than 1% of cases, has a fulminant course with the development of acute liver failure (2, 3). Other complications of HAV infection include acute kidney injury (AKI), autoimmune hemolytic anemia, aplastic anemia, acute pancreatitis, mononeuritis, reactive arthritis, glomerulonephritis, cryoglobulinemia, Guillain-Barré syndrome, and pleural or pericardial effusion (4). Although extrahepatic manifestations are uncommon in hepatitis A, several authors have reported the development of AKI in 7.2% of patients with HAV infection (5). Here, we present a clinical case of severe hepatitis A, emphasizing the importance of identifying rare extrahepatic complications in HAV patients. The present study aimed to describe a clinical case of severe viral hepatitis A complicated by AKI.
2. Materials and Methods
Biochemical parameters — alanine transaminase (ALT) and aspartate aminotransferase (AST), creatinine and urea, bilirubin, gamma-glutamyl transferase (GGT) and alkaline phosphatase, calcium ions, total protein and albumin, C-reactive protein — were tested on the ARCHITECT ci8200 analyzer, USA. Anti-HAV Immunoglobulin M (IgM) antibodies were detected in the patient’s serum sample using a commercially available ELISA Vectohep A-IgM kit (Vector-Best, Novosibirsk, Russia). Hepatitis A virus ribonucleic acid (RNA) testing in the serum sample was performed using a commercially available RT-PCR kit, AmpliSens HAV-FRT PCR kit (Central Research Institute of Epidemiology, Moscow, Russia). Hepatitis A virus genotype was determined based on phylogenetic analysis of the VP1/2A region. For this purpose, the corresponding fragment of the HAV genome was amplified from the serum sample with primers and protocol described previously (6). The amplified fragment of the HAV genome was purified from agarose gel using a QIAquick Gel Extraction Kit (QIAGEN, Hilden, Germany) and sequenced in a 3130 Genetic Analyzer (ABI, Foster City, CA, USA) automatic sequencer using the BigDye Terminator v3.1 Cycle Sequencing Kit. Hepatitis A virus sequence was subjected to phylogenetic analysis using the maximum likelihood (ML) method in the IQ-TREE software (v.2.3.6), together with the HAV sequences obtained in different areas of Russia in recent years. Antibodies to hepatitis E virus (anti-HEV) IgM and IgG were tested in the patient’s serum using commercial ELISA tests: DS-EIA-ANTI-HEV-M and DS-EIA-ANTI-HEV-G, respectively (Diagnostic Systems, Nizhny Novgorod, Russia). The HEV RNA testing was performed using an in-house PCR assay with primers to the open reading frame 2 (ORF2) fragment of the HEV genome, as described elsewhere (7). Hepatitis B and C serological markers (HBsAg, HBeAg, anti-HBc IgM, anti-HBe, anti-HCV) were tested in the patient’s serum using commercially available ELISA kits (Vector-Best, Novosibirsk, Russia). The HBV deoxyribonucleic acid (DNA) and HCV RNA were tested in plasma using commercial PCR kits (Central Research Institute of Epidemiology, Moscow, Russia) with a limit of detection of 10 IU/mL and 25 IU/mL, respectively. The RNA of SARS-CoV-2 and influenza A, B, and acute respiratory viruses was tested in a nasopharyngeal swab sample using commercial RT-PCR kits (Central Research Institute of Epidemiology, Moscow, Russia). The DNA of leptospira, parvovirus B19, herpes simplex virus 1, 2, Epstein-Barr virus (EBV), and cytomegalovirus (CMV), as well as hantavirus RNA, were tested in blood plasma using commercial PCR kits (Central Research Institute of Epidemiology, Moscow, Russia). Direct hemagglutination tests with Yersinia for pseudotuberculosis diagnosis (Ekolab, Elektrogorsk, Russia), an enzyme immunoassay to detect immunoglobulins M and G against leptospirosis pathogens (Omnix ("Helix"), St. Petersburg, Russia), and an immunofluorescence test for hemorrhagic fever with renal syndrome (HFRS) were performed in the serum sample using commercially available kits (Polio Institute named after M.P. Chumakov, Moscow, Russia). All testing with commercial kits was performed according to the instructions of the manufacturers of the respective kits.
3. Case Presentation
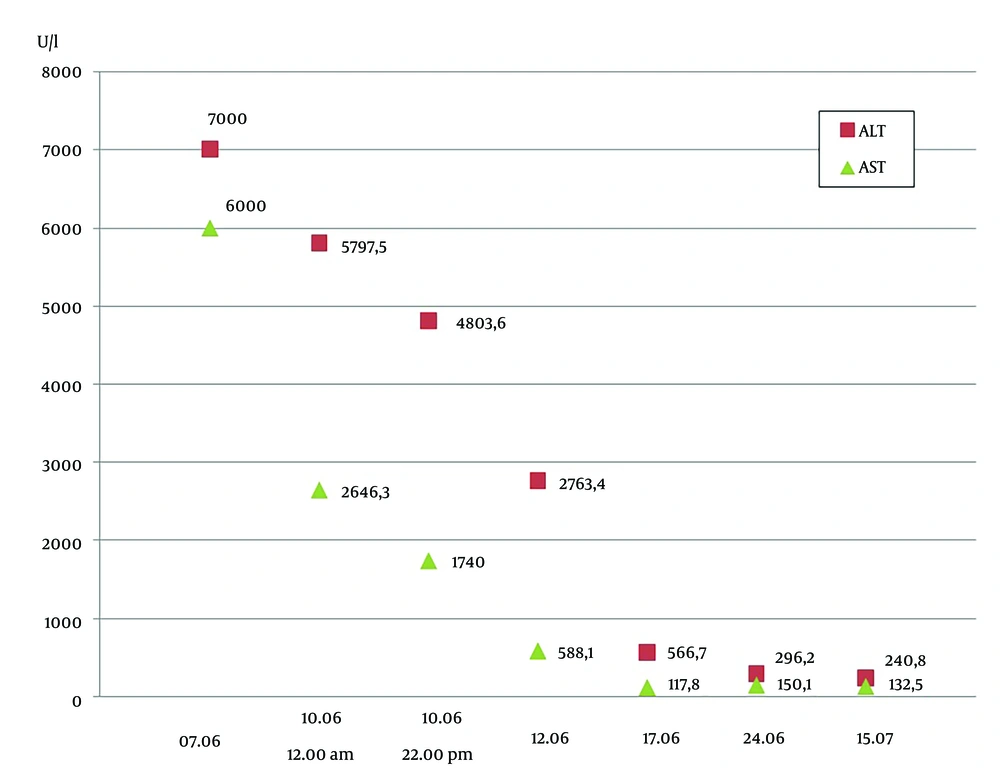
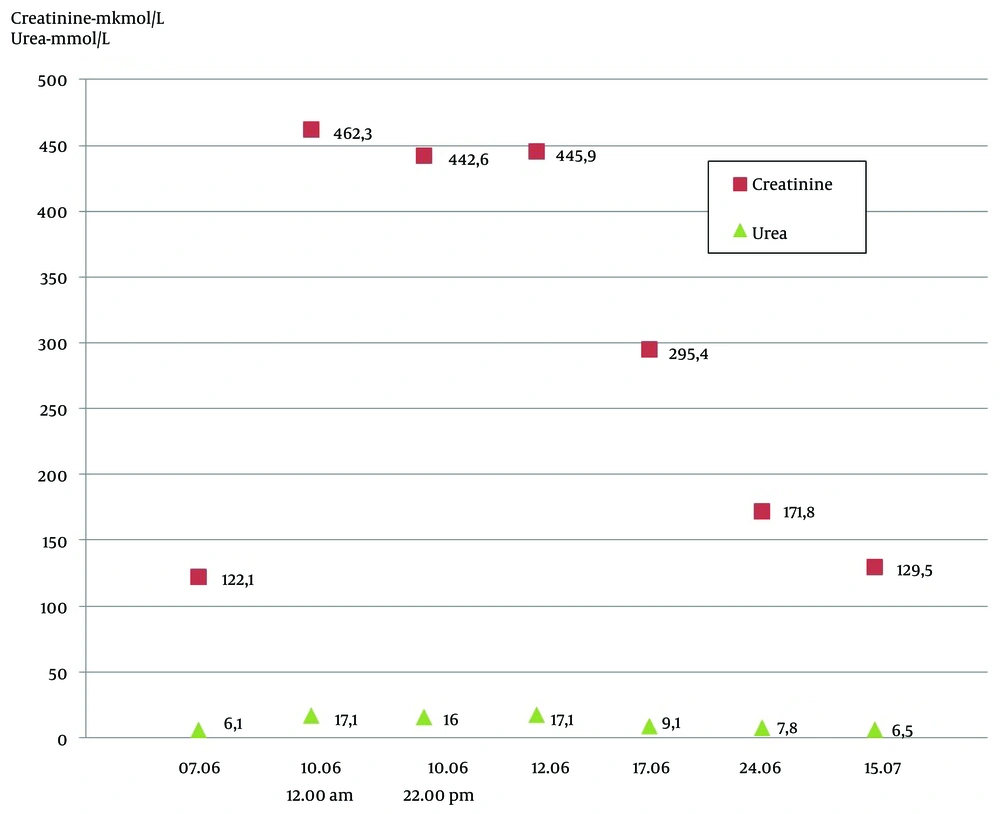
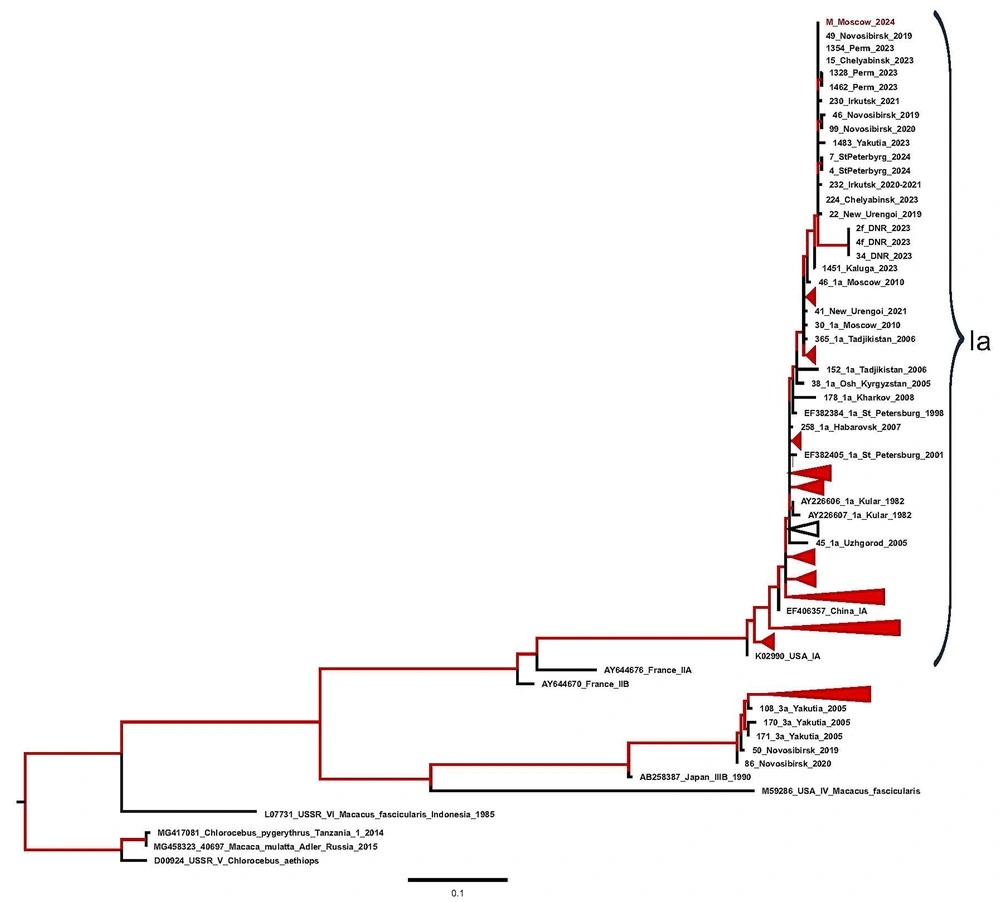
A 34-year-old man was admitted to the infection hospital in Moscow on the 4th day of the illness with a preliminary diagnosis of an acute respiratory viral infection. His symptoms included weakness, fever, nausea, and loss of appetite. From May 4 to May 12, 2024, the patient traveled around the Ural region, lived in a tent, kayaked on the Ural River, and drank non-boiled water from mountain streams. He had a tooth extraction in April 2024. He denied any significant medical, family, or social history. The patient had fallen ill suddenly, with his temperature increasing to 37.5°C, marked weakness, and myalgia. He began taking paracetamol (2 - 3 tablets per day) and ibuprofen (2 - 3 tablets per day) with short-term effects, and his maximum body temperature increased to 40°C. He was examined by a polyclinic doctor on the 3rd day of the disease, and sodium metamizole with papaverine was prescribed, without effect. The next day, on June 7, the patient was admitted to the clinic. On June 8 and 9, he experienced vomiting, heaviness in the right hypochondrium, and anorexia. On admission, his condition was of moderate severity. He had clear consciousness, answered questions adequately, and was well-oriented. Meningeal and focal neurological symptoms were not observed. His vital signs were normal. Laboratory tests revealed leukopenia (3.0 × 109/L) with lymphopenia (0.60 × 109/L, 19%) and monocytopenia (0.2 × 109/L, 7.3%), and thrombocytopenia (114 × 109/L). A biochemical blood test showed elevated levels of ALT and AST. Laboratory results (AST and ALT) obtained during hospitalization and post-discharge follow-up are shown in Figure 1. The total bilirubin was increased to 2 upper limits of normal (42.3 mmol/L), GGT and alkaline phosphatase were increased (291.7 U/L and 138.0 U/L, respectively), calcium ions were low at 1.04 mmol/L, the level of total protein was low at 61.4 g/L with normal albumin levels (37.9 g/L), C-reactive protein was increased at 73.1 mg/L, and creatinine was slightly higher than the reference range. The dynamics of creatinine and urea levels during hospitalization and post-discharge follow-up are shown in Figure 2. In the coagulogram, the concentration of prothrombin dropped to 36%, the prothrombin time, INR, and aPTT were higher than the reference range (23.1 s, 2.050, and 44.1, respectively). The general analysis of urine revealed proteinuria (0.7 g/L) and hematuria (10,000 quantity/µL). In the following days, the patient worsened. On June 8 (5th day of disease), the patient started vomiting, dizziness, heartburn, and abdominal discomfort and lost appetite. On objective examination, icteric skin and sclera, urine color change to bright yellow, and the liver protruding 2.0 cm from under the edge of the costal arch were noted. The PCR tests for SARS-CoV-2 RNA and influenza A, B, and acute respiratory viruses were negative. Markers of hepatitis B and C (HBsAg, HBeAg, anti-HBc IgM, anti-HBe, anti-HCV, HBV DNA, HCV RNA) were not detected. Anti-HAV IgM antibodies and HAV RNA were detected in the patient’s serum sample using commercially available diagnostic tests. Hepatitis A virus genotype IA was identified based on phylogenetic analysis of partial VP1/2A sequence amplified from the patient’s specimen. The obtained HAV sequence clustered with other HAV IA sequences from sporadic cases identified in Russia throughout 2019 - 2023 (Figure 3). Considering the acute onset of the disease that had an influenza-like variant, severe intoxication syndrome, severe cytolytic syndrome, hemorrhagic syndrome (prothrombin concentrations 36%), and the presence of anti-HAV IgM antibodies and HAV RNA, the following diagnosis was made: Hepatitis A, icteric form, severe course. Basic disintoxication therapy was prescribed: Electrolytes and colloid solutions, lactulose, rifaximin, and omeprazole (Table 1). On the 7th day of the disease, the patient developed a nasal hemorrhagic discharge; laboratory tests revealed creatinine and urea were significantly higher, total bilirubin was increased to 103.5 mmol/L mainly due to the direct fraction (direct bilirubin 66.4 mmol/L), alkaline phosphatase, GGT, and lactate dehydrogenase were higher than the reference range (282 U/L, 480 U/L, and 675 units/L, respectively), potassium ions were increased at 7.95 mmol/L and sodium and calcium ions were decreased (128 mmol/L and 0.86 mmol/L, respectively). There was a tendency to decrease C-reactive protein to 33.5 mg/L. In the hemogram, leukocytosis (11.2 × 109/L), lymphomonocytosis (lymphocytes 7.1 × 109/L, 63% and monocytes 1.2 × 109/L, 10.6%). Positive dynamics were observed in the coagulogram-the concentration of prothrombin increased to 61%, and the prothrombin time, INR, and aPTT were below the reference range (15.4 s, 1.390, and 30.2, respectively). An ultrasound examination of the abdominal cavity showed increasing and diffuse changes in the parenchyma of the liver and kidneys. The CT scans of the chest organs showed minimal hydropericardium. High levels of creatinine (over 300 mmol/L) and urea, hyperkalemia, proteinuria, and hematuria, and the travel history to an endemic region for HFRS gave a reason to assume the presence of a mixed infection in the patient and to examine for leptospirosis and HFRS. Therefore, a comprehensive examination by PCR was performed for hantavirus RNA in blood plasma, as well as DNA of leptospira, parvovirus B19, herpes simplex virus 1, 2, EBV, and CMV in blood plasma; none of these pathogens was detected. Direct hemagglutination test with Yersinia for pseudotuberculosis diagnosis, the microagglutination reaction for leptospirosis, and immunofluorescence assay for HFRS virus were negative. The DNA of EBV was detected (8.15 × 102 copies/mL). Considering the development of complications in the hepatitis A patient, such as acute hepatic encephalopathy stage I and acute renal failure, extracorporeal hemocorrection was performed in the intensive care unit. Amino acid solutions were added to therapy intravenously, ornithine per 200 mL of 5% glucose solution, furosemide, rabeprazole (Table 1). After extracorporeal hemocorrection, the concentration of potassium ions decreased to the reference range (3.6 mmol/L), calcium ions increased to 1.0 mmol/L, and creatinine and urea decreased slightly. Total protein and albumin were decreased (57.7 g/L and 31.4 g/L, respectively). The general urine test was normal. On the 2nd day after extracorporeal hemocorrection, the patient’s condition stabilized and he was transferred to the specialized infectious diseases department for further treatment. On the 3rd day after plasma exchange (day 9 of the disease), the patient’s condition remained severe, weakness increased, diuresis decreased, and a feeling of "swelling in the body" appeared, along with heaviness in the chest. The patient was able to communicate but answered questions monosyllabically. Peripheral edema of shins and feet was noted. Kidney effleurage symptom was negative on both sides. Despite a decrease in cytolysis, the levels of residual protein metabolism products and potassium (5.55 mmol/L) were higher, while the levels of total protein (54.3 g/L), albumin (30.6 g/L), and prothrombin concentration (66%) were low. In the days following pathogenetic therapy, the patient’s condition was severe but stable, with a moderate amount of free fluid in the abdominal cavity and pleural cavities. Impaired protein-synthetic liver function persisted (total protein 48.4 g/L, albumin 24.6 g/L, prothrombin concentration 46%). There was a slow positive decrease in creatinine and urea levels, cytolysis indicators decreased, bilirubin increased (total bilirubin in the blood was 218.5 mmol/L, bilirubin in urine was 8.5 mmol/L), pyuria (170,000 leukocytes), hematuria (4,400 erythrocytes), and moderate cylindruria (60 cylinders) in the analysis of urine according to Nechiporenko. No microorganism growth was observed in bacteriological urine culture. Since the patient returned from the region where sporadic cases of viral hepatitis E were reported (Republic of Bashkiria), as well as a slow recovery after extracorporeal hemocorrection of glomerular filtration of the kidneys on the 11th day of the disease, an examination for HEV RNA and anti-HEV IgM and IgG antibodies was performed. At initial testing, the patient’s serum was reactive for anti-HEV IgM antibodies, but negative for anti-HEV IgG and HEV RNA. On repeated testing on the 40th day of the disease, anti-HEV IgM antibodies were not detected, as well as anti-HEV IgG antibodies and HEV RNA, indicating nonspecific reactivity for anti-HEV IgM antibodies at initial testing. The results obtained made it possible to exclude the diagnosis of viral hepatitis E. Following the examination results, the patient was clinically diagnosed with: Hepatitis A, icteric form, severe course. Complications: Acute hepatic encephalopathy stage I, acute renal failure. On the 15th day of the disease, the patient’s condition was severe, but stable, with creatinine at 295.4 µmol/L, urea 9.1 mmol/L, prothrombin concentration 38%. On the 21st day of the disease, the patient’s condition was assessed as of moderate severity. Weakness persisted, total protein (66.7 g/L) and albumin (34.4 g/L) increased to the reference ranges, and the concentration of prothrombin increased to 45.2%. The patient was discharged in satisfactory condition on the 44th day of disease (on the 39th day of hospitalization) under the supervision of specialist doctors at his place of residence with pronounced positive dynamics of laboratory parameters. On objective examination, the skin was icteric and there was no pastiness of the shins and abdominal edema.
Maximum likelihood (ML) phylogenetic tree for the hepatitis A virus (HAV) VP1/2A sequences. For each sequence, the number in the GenBank database, the country, the city (in the case of sequences from Russia), and the year of isolation are indicated. Sequence from this study (indicated with prefix “A case” in the name of sequence) is shown in red with the indicated year of isolation. The tree branches shown in red have a posterior probability of > 90%.
| Drug Names | Dosage | Administration Routes | Treatment Duration (d) |
|---|---|---|---|
| 5% dextrose injection | 500 mL | IV once a day in the morning | 39 |
| 0.9% sodium chloride injection | 1000 mL | IV once a day in the morning | 40 |
| Rifaximin tablets | 200 mg | Per os 3 times a day | 16 |
| Lactulose solution | 15 mg | Per os 3 times a day | 41 |
| Omeprazole lyophilisate for solution for infusions 40 mg | 40 mg | IV once a day in the morning | 7 |
| Omeprazole capsules | 20 mg | Per os 2 times a day | 22 |
| Rabeprazolecapsules | 40 mg | Per os 2 times a day | 19 |
| Amino acids for parenteral nutrition infusion solution | 500 mL | IV once a day | 3 |
| L-ornithine L-aspartate infusion concentrate | 40000 mg | IV once a day | 2 |
| 1% furosemide injection | 40 mg | IV once a day | 5 |
| 20 mg | IV once a day | 7 | |
| IM once a day | 5 |
Treatment Patient (Drug Names, Dosage, Treatment Duration and Intervals)
4. Discussion
Although the course of hepatitis A is favorable in most cases, the risk of developing fulminant liver failure and a fatal outcome is possible, especially in adults, immunocompromised patients, or those with underlying hepatic diseases (8, 9). However, kidney-related complications are described even in patients with non-fulminant hepatitis A (10, 11). Several hypotheses have been proposed regarding the pathogenesis of nephropathy during hepatitis A: Pre-renal factors such as vomiting or diarrhea responsible for hypovolemia and activation of the renin-angiotensin-aldosterone system; toxic effect of bile acid salts on the renal tubules. In addition, hyperbilirubinemia is believed to cause a decrease in systemic vascular resistance, narrowing of the renal vessels, and impaired contractility of the left ventricle. The direct cytotoxic effect of HAV has also been reported, as in viral hepatitis B and C. Earlier, back in the 1970s - 1990s, the mechanism of formation of immune complexes in hepatitis A was discussed (12, 13). Therefore, AKI in hepatitis A can also occur in people without a comorbid background. The Epstein-Barr virus detected in the blood serum does not lead to acute kidney damage, as it does not infect them. The detection of Epstein-Barr DNA in low load indicates its reactivation against the background of severe hepatitis A. This condition is not only associated with risks of a severe or even fulminant course of this disease, but also with the risk of the transition of AKI to chronic kidney disease, which also requires a revision of the follow-up period after hepatitis A convalescence and the expansion of the list of mandatory laboratory tests during the follow-up. This clinical case highlights the rare but significant association between the fulminant course of hepatitis A and the development of an extrahepatic complication of AKI, which leads to prerenal azotemia or the development of immune complex-mediated glomerulonephritis (14).
4.1. Conclusions
This clinical case is of practical interest for doctors of various specialties (internists, general practitioners, nephrologists, and infectious disease specialists), as it demonstrates the differential diagnostic search in a patient with the leading clinical syndrome — AKI. Considering that acute liver failure is the well-known and pathogen-related complication of acute viral hepatitis, clinicians may not associate the symptoms of AKI identified during the patient’s examination with HAV infection, which may lead to misdiagnosis.