1. Context
Acute bacterial rhinosinusitis (ABRS), defined as inflammation of the paranasal sinuses, manifests in two primary forms: Acute (lasting less than 4 weeks) and chronic (lasting over 12 weeks) (1). The clinical manifestation demonstrates substantial variation, ranging from slight facial pressure and troublesome nasal congestion to more severe facial discomfort, fatigue, and even fevers (1). Sinusitis affects approximately 15% of the population each year, with higher rates observed in women compared to men (2, 3). Research indicates a relatively high frequency of ABRS in Iran, estimated at about 53%. Maxillary sinusitis is the most frequent type. However, there’s a lack of data on the exact incidence of ABRS, which is likely lower than the general sinusitis prevalence (4).
1.1. Risk Factors for Acute Bacterial Rhinosinusitis
Older age, viral upper respiratory tract infections, smoking, flight travel, swimming, exposure to atmospheric pressure variations (including activities like deep-sea diving), allergies and asthma, dental diseases, and immunodeficiency are risk factors for ABRS (5).
1.2. Microbiology
The etiology of sinusitis can be bacterial, viral, or fungal. Most instances of acute sinusitis are viral, especially rhinoviruses. In the order listed, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are the most often found bacterial species in sinus aspirates from both adults and children (1, 6).
1.3. Scope and Purpose
This Iranian guideline aims to standardize antimicrobial treatment and patient care for ABRS in the general population through evidence-based management strategies. Due to significant regional variations in bacterial etiology, antibiotic resistance patterns, drug availability, healthcare infrastructure, and resource constraints, the need for geographically specific guidelines is emphasized. Overall, these guidelines equip healthcare professionals with the tools to make informed decisions regarding initial empiric antibiotic therapy and subsequent evaluation of infectious etiology in ABRS management.
2. Method
A team of experts from various disciplines reviewed relevant data published between January 1990 and April 2024. This data came from Iranian databases (IranMedex, Irandoc, MagIran) and international sources (Google Scholar, Scopus, PubMed, SID). They also included studies from PubMed to ensure comprehensive coverage. The review focused on studies from Iran that investigated the prevalence, serotype distribution, and antibiotic resistance patterns of key bacterial pathogens involved in ABRS. These pathogens included S. pneumoniae, M. catarrhalis, and H. influenzae. The studies analyzed clinical samples from various sources relevant to ABRS, such as the nasopharynx, ears, eyes, and sinuses. Carrier studies analyzing nasopharyngeal specimens were also included.
The expert panel employed a structured, iterative consensus-building process using a modified Delphi technique: Multidisciplinary specialists (infectious disease specialists and clinical pharmacists) with ≥ 5 years of experience first reviewed evidence from Iranian and international databases to draft preliminary recommendations. Anonymous voting identified areas of disagreement (consensus threshold: ≥ 70% agreement), followed by moderated virtual discussions to reconcile conflicting views, prioritizing local Iranian data (e.g., antibiotic resistance patterns) and grading of recommendations, assessment, development, and evaluation (GRADE) criteria for clinical relevance. Recommendations underwent final anonymous voting, with unresolved items deferred or excluded.
2.1. Grading of Guideline Recommendations
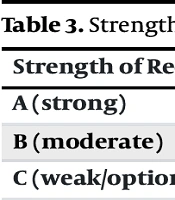
We utilized the GRADE criteria alongside expert opinion to evaluate the evidence for each recommendation (7). The PICO analysis is summarized in Table 1. The definitions for the quality of evidence used in the assessment are detailed in Table 2, and the framework for assigning the strength of recommendation is provided in Table 3.
| Author/Year | Population | Number of Subjects | Intervention and Control | Outcome Measures | Results | Type of Test | Study Methodology | GRADE Assessment |
|---|---|---|---|---|---|---|---|---|
| Amiri et al. (2015), (4) | Iranian population with sinusitis | 1,057 | Meta-analysis of studies | Prevalence of various types of sinusitis | Overall prevalence: 53% (CI 40% - 65%), maxillary: 68%, frontal: 17%, ethmoid: 31%, sphenoid: 19% | N. A. | Systematic review and meta-analysis | 1 |
| Hatami et al. (2024), (8) | Haemophilus influenzae and Moraxella catarrhalis isolates in Iran | 189 isolates | Systematic review of antibiotic resistance patterns | Antibiotic resistance patterns of H. influenzae and M. catarrhalis | H. influenzae: High resistance to ampicillin, M. catarrhalis: High resistance to penicillin | N. A. | Systematic review | 2 |
| Khademi and Sahebkar (2021), (9) | Streptococcus pneumoniae isolates from Iran | 1,249 reports from 58 studies | Meta-analysis of studies | Prevalence of penicillin-nonsusceptible and multidrug-resistant S. pneumoniae | PNSP: 46.9%, MDR: 45.3%, high resistance to erythromycin, azithromycin | N. A. | Systematic review and meta-analysis | 1 |
| Khoramrooz et al. (2012), (10) | Iranian children with OME | 15 centers | 48 OME patients (63 middle ear fluid samples, 48 adenoid tissues) | PCR and bacterial culture methods for pathogen detection | Bacterial detection: Alloicoccus otitidis: 23.8% (culture), 36.5% (PCR)-S. pneumoniae: 35.5% (adenoid culture), 31.2% (PCR) antimicrobial susceptibility: Most isolates sensitive to ampicillin, amoxicillin/clavulanate, fluoroquinolones | Disc diffusion method | Cross-sectional observational study | 4 |
| Eghbali et al. (2020), (11) | Patients with respiratory tract infections in northern Iran | 280 patients, 120 controls | Isolation and susceptibility testing of M. catarrhalis | Prevalence of M. catarrhalis and antibiotic resistance patterns | Resistance to penicillin, presence of β-lactamase, various resistance genes detected | Disc diffusion method | Observational study | 4 |
| Farajzadeh Sheikh et al. (2021), (12) | Patients with CAP in southwest Iran | 92 sputum samples | Detection of S. pneumoniae and H. influenzae using culture and M-PCR methods | Detection rates and antibiotic resistance patterns of S. pneumoniae and H. influenzae | Detection rates (culture): S. pneumoniae: 16.3%; H. influenzae: 7.6%; detection rates (M-PCR): S. pneumoniae: 35.8%; H. influenzae: 11.9%; Antibiotic resistance: S. pneumoniae: 13.3% resistant to ceftriaxone; H. influenzae: 28.6% resistant to clarithromycin, ceftriaxone, gentamicin | Disc diffusion method | Cross-sectional observational study | 4 |
| Yousefi et al. (2021), (13) | S. pneumoniae isolates in Iran | 33 studies (total isolates not specified) | Systematic review of serotype distribution and resistance patterns | Frequency of S. pneumoniae, serotype distribution, and antimicrobial resistance patterns | High resistance to co-trimoxazole, penicillin, erythromycin, common serotypes: 23F, 19F | N. A. | Systematic review | 4 |
| Shokouhi et al. (2019), (14) | S. pneumoniae isolates in Iran | 2,723 cases across 25 studies | Review of macrolide resistance patterns | Resistance patterns and mechanisms of S. pneumoniae to macrolides | Mean macrolide resistance: 48.43%, ermB and mefA mutations prevalent | N. A. | Narrative review | 4 |
| Kargar et al. (2014), (15) | S. pneumoniae isolates from hospitals in Iran | 82 isolates | PCR-RFLP analysis of quinolone resistance-determining regions | Presence of mutations in quinolone resistance genes and antibiotic susceptibility | Mutation rates: parC: 75.56%, gyrA: 68.89%, high resistance to nalidixic acid | Disc diffusion method | Observational study | 4 |
| Beheshti et al. (2020), (16) | S. pneumoniae isolates from clinical samples in Tehran, Iran | 44 invasive isolates | Analysis of antibiotic resistance and molecular characterization | Resistance patterns, capsular types, and genetic diversity of S. pneumoniae | High erythromycin resistance (73%), MDR in penicillin-resistant strains, common types: 6A/B, 19A | Disc diffusion method | Observational study | 4 |
| Mohammadi Gharibani et al. (2019), (17) | Healthy children in Ardabil, Iran | 43 isolates | Analysis of antibiotic resistance and resistance mechanisms | Antibiotic resistance patterns and genetic mechanisms of macrolide resistance | High macrolide resistance: Erythromycin 74.4%, genetic: 100% mefA/E, 81.25% ermB | E-test strips method | Cross-sectional observational study | 4 |
| Shooraj et al. (2019), (18) | Children under 6 years old in Iran | 328 nasopharynx swabs | Analysis of clonal diversity and antibiotic resistance | Prevalence, antibiotic resistance patterns, and clonal diversity of H. influenzae | 73 strains of H. influenzae, 42% resistance to chloramphenicol, 43% to ampicillin, 28 PFGE patterns | Disc diffusion method | Cross-sectional observational study | 4 |
Abbreviations: GRADE, grading of recommendations, assessment, development, and evaluation; PNSP, penicillin-nonsusceptible Streptococcus pneumoniae; OME, otitis media with effusion; CAP, community-acquired pneumonia.
| Quality of Evidence | Description | Source of Evidence |
|---|---|---|
| 1 | High confidence that the true effect lies close to that of the estimate of the effect. | Evidence from multiple well-conducted RCTs or meta-analyses of RCTs. |
| 2 | The true effect is likely to be close to the estimate, but there is a possibility that it is different. | Evidence from one or more RCTs with limitations or strong evidence from well-designed observational studies. |
| 3 | The true effect may be substantially different from the estimate. | Evidence from well-conducted cohort or case-control studies, or downgraded RCTs with significant limitations. |
| 4 | The true effect is likely to be substantially different from the estimate. | Evidence from observational studies with significant limitations, non-randomized studies, or expert opinion. |
| 5 | Any estimate of effect is very uncertain. | Evidence from unsystematic clinical observations, case reports, or expert opinion without strong supporting data. |
Abbreviation: RCTs, randomized controlled trials.
| Strength of Recommendation | Descriptions |
|---|---|
| A (strong) | The benefits of the recommended intervention clearly outweigh the risks. High confidence in its efficacy. |
| B (moderate) | The benefits of the intervention outweigh the risks, but there is less certainty about the balance of benefits and risks. |
| C (weak/optional) | The balance between benefits and risks is uncertain or close, making the recommendation more context-dependent. |
3. Recommendations
3.1. Which Antibiotics are Recommended as First-Line Empiric Therapy for Adults with Uncomplicated Acute Bacterial Rhinosinusitis?
The primary course of treatment for the majority of patients diagnosed with ABRS should consider the significant resistance rates observed in Iran.
- Amoxicillin-clavulanate (500 mg/125 mg orally three times daily or 875 mg/125 mg orally twice daily) is recommended as first-line therapy (1A).
- High-dose amoxicillin-clavulanate (2000 mg/125 mg extended-release tablets orally twice daily) is recommended as first-line therapy in cases where there is a significant concern about antibiotic resistance, particularly due to the high prevalence of penicillin-nonsusceptible S. pneumoniae (2A).
- The administration of β-lactams and/or co-trimoxazole would not have the desired therapeutic effect (3C).
- Monotherapy with clindamycin, 3rd generation cephalosporin, or doxycycline is not recommended (2B).
- Macrolides monotherapy is not recommended for empirical treatment due to high resistance rates among S. pneumoniae and potential methylation and efflux-mediated resistance (2B).
- To overcome resistance to H. influenzae and M. catarrhalis, a macrolide can also be added (3C).
3.2. Which Antibiotic is Recommended for Adults with Uncomplicated Acute Bacterial Rhinosinusitis Who Experience Penicillin Allergy?
- For adults diagnosed with uncomplicated ABRS who have a penicillin allergy, a respiratory fluoroquinolone such as levofloxacin (750 mg or 500 mg orally once daily) or moxifloxacin (400 mg orally once daily) for 5 to 7 days is recommended as an alternative for empiric antimicrobial therapy (3C).
- If an immediate-type hypersensitivity response is confirmed through skin testing, which is strongly advised for patients with a questionable history of penicillin allergy, treatment with respiratory fluoroquinolones is recommended (4C).
- Macrolides and TMP/SMX, previously used for patients allergic to penicillin, are no longer recommended due to increasing resistance among penicillin-nonsusceptible S. pneumoniae (2B).
- If monitoring and facilities for outpatient therapy are accessible, intravenous 3rd generation cephalosporin with close monitoring could be recommended for patients with penicillin intolerance/non-Type I hypersensitivity reactions (3C).
- TMP/SMX and macrolides are not recommended unless the patient is β-lactam allergic due to limited effectiveness against major ABRS pathogens and possible bacterial failure (2B).
3.3. Which Antibiotic is Recommended for Adults with Uncomplicated Acute Bacterial Rhinosinusitis Who Experience Treatment Failure?
- For adults diagnosed with uncomplicated ABRS who experience treatment failure, a change in management is necessary. This is defined as the patient not improving or worsening within 7 days of diagnosis. If there is no response to antimicrobial therapy after 72 hours, either switching to a different antibiotic or re-evaluating the patient is recommended (3C).
- For patients initially managed with observation and later experiencing treatment failure, starting treatment with high-dose amoxicillin with clavulanate is advised (2B).
- Penicillin-allergic patients should consider using a respiratory fluoroquinolone like levofloxacin or moxifloxacin (2B).
- In adults with a history of non-type I hypersensitivity to penicillin, ceftriaxone may be appropriate (3C).
- Fluoroquinolones (levofloxacin or moxifloxacin) should be reserved for cases with known resistance or treatment failure to avoid promoting further resistance (2B).
- If monitoring and facilities for outpatient therapy are accessible, 3rd generation cephalosporin with close monitoring could be recommended (4C).
- For patients who do not respond to initial treatment, initiating therapy with cefixime and clindamycin is recommended (4C).
4. Summary of Evidence
In Iran, S. pneumoniae exhibits varying levels of antibiotic resistance, including resistance to amoxicillin. Studies indicate a high prevalence of penicillin-nonsusceptible S. pneumoniae (PNSP) strains, with amoxicillin resistance rates reaching 30.5% (9). Living in regions where PNSP rates exceed 10% poses a significant risk factor for pneumococcal resistance (19). Additionally, due to the production of β-lactamase by M. catarrhalis and H. influenzae, amoxicillin proves ineffective against these pathogens, thus it is not recommended as a first-line therapy in Iran. In line with regional trends, high rates of amoxicillin resistance have been observed in M. catarrhalis isolates, with studies reporting resistance rates of 100% and 81.2% (10, 11). These findings suggest amoxicillin may not be a suitable first-line therapy for M. catarrhalis infections. While the prevalence of β-lactamase-producing H. influenzae in the United States ranges from 27% to 43% and is not expected to respond to amoxicillin without clavulanate (20), resistance to amoxicillin-clavulanate is very high in Iran; specifically, the antibiotic resistance to amoxicillin-clavulanate for H. influenzae in patients with community-acquired pneumonia is around 85.7%. However, this resistance rate cannot be extrapolated to patients with ABRS (12). In Iran, studies have extensively investigated the resistance patterns of S. pneumoniae to various antibiotics, but there is no specific mention of amoxicillin-clavulanate resistance in the provided contexts. Among respiratory pathogens in Iran, ceftriaxone demonstrated the most favorable resistance profile, with resistance rates of 13.3% for S. pneumoniae, 28.6% for H. influenzae, and 6.2% for M. catarrhalis isolates (8, 12, 13). Consistent with prior reports of geographically variable macrolide resistance (10% - 100%), one analysis of 25 studies (n = 2723) identified a mean resistance rate of 48.43% (CI, 38.8 - 57.9%) (14); additionally, macrolides showed efficacy against both H. influenzae and M. catarrhalis isolates, highlighting their potential as effective therapeutic options (8). Fluoroquinolone resistance in S. pneumoniae is a concerning issue in Iran, as studies have shown a significant correlation between quinolone resistance development and mutations in the parE, parC, and gyrA genes (15). Studies conducted in Iran have demonstrated heterogeneity in ciprofloxacin susceptibility among respiratory pathogens. Streptococcus pneumoniae exhibits the lowest resistance rates (0 - 11%), whereas M. catarrhalis (0 - 70%) and H. influenzae (0 - 57.1%) display a wider range of susceptibility (8, 11, 12, 21). Studies investigating levofloxacin resistance in S. pneumoniae from Iran have documented regional variations. Research in Tehran found a low prevalence (2%) of levofloxacin-resistant invasive S. pneumoniae isolates (16). Further supporting this trend, a separate cross-sectional study involving 43 isolates of S. pneumoniae from healthy children in Ardabil reported no resistance to levofloxacin (17). Similarly, studies evaluating fluoroquinolone susceptibility among respiratory pathogens, including M. catarrhalis and H. influenzae, observed a 0% resistance rate to levofloxacin (10, 12, 18). These findings collectively suggest potentially low levels of levofloxacin resistance in S. pneumoniae and some other respiratory bacteria associated with ABRS in Iran. Furthermore, levofloxacin resistance in Iranian children was found to be 0.8% and 1.7% for S. pneumoniae, respectively, based on a subgroup analysis of 27 studies (9). In a cross-sectional study conducted in Ardabil, antibiotic resistance profiles of 43 S. pneumoniae isolates from healthy children were determined using the disk diffusion method. Clindamycin resistance was identified in 28% of isolates, with no evidence of inducible resistance (17). Unfortunately, direct assessment of doxycycline resistance among S. pneumonia in Iran remains a topic for future investigation.
5. Research Needed in Iran
There is a notable scarcity of head-to-head randomized clinical trials and robust evidence concerning the management of patients with ABRS. Additionally, there is a pressing need for clinical trials that juxtapose different antimicrobial treatment protocols for outpatient settings. These trials should thoroughly evaluate the occurrence of adverse effects associated with antibiotics. It is imperative to disseminate the findings of antibiograms featuring broad-spectrum antibiotics and to gauge the prevalence of particular pathogens to enhance the detection of antimicrobial susceptibility. Moreover, research into the antimicrobial resistance of antibiotics like clindamycin and doxycycline against ABRS pathogens is scarce and should be explored.