1. Background
In 1983, presence of a gram-negative, spiral and microaerophilic bacterium in patients with gastritis and peptic ulcer was reported by three different research groups in the literature. This spiral bacterium was first called “Campylobacter-like organism” but after a while it was renamed as Helicobacter pylori (H. pylori) (1). H. pylori is usually present either in the deep layers of lining mucosa of gastric epithelium or between those layers and gastric epithelium (2). This organism is adapted for colonization in the human stomach and exists in almost half of the human population, but different species of H. pylori cause increased risk of diseases like gastritis and thereinafter gastric ulcer, duodenal ulcer, gastric cancer and mucosal associated lymphoid tissue (MALT) lymphoma by their pathogenic factors such as urease, BabA, OipA, CagPA1, SabA and VacA (3, 4).
The term “gastritis” is used when inflammation of the gastric mucosal layer is confirmed histologically. This disease can be categorized based on the duration (acute or chronic gastritis), histologic features, anatomical distribution and possible mechanisms of pathogenecity. At present, it is believed that any factor causing primary lesion in gastric mucosal layer can lead to gastritis and gastric atrophy (in chronic conditions), which is considered an important risk factor for gastric cancer (5). There are wide and heterogenous factors for incidence of gastritis including biliary reflux into the stomach, non-steroidal anti-inflammatory drugs (NSAIDs), stress, inappropriate diet, cigarette smoking, severe anemia and immune system disorders. Many previous studies have been conducted to determine the prevalence of H. pylori in the general population and patients with upper gastrointestinal problems including gastritis. Based on their results, prevalence of H. pylori differs among populations in the world and is strongly correlated with life standards like socio-economic status, educational level, and health condition (6, 7). Prevalence of H. pylori infection in Iranian adults was reported at up to 80% based on serological studies (8).
H. pylori eradication is of clinical importance to decrease gastric inflammatory diseases and to prevent progression towards carcinoma. Multi-drug regimens have been proposed as the most effective therapy. Examples are: 1) three-drug regimen: omeprazole, clarithromycin, amoxicillin or metronidazole; 2) four-drug regimen: a bismuth derivative, metronidazole, tetracycline and a proton-pump inhibitor. One of the most important reasons for H. pylori treatment failure is the ever-growing antibiotic resistance resulting in lack of treatment efficacy particularly in developing countries (9-11).
2. Objectives
Regarding the importance of H. pylori infection and resistance to current regimens, this study was conducted to determine the prevalence of H. pylori infection in patients with gastritis, referred to Shahid Beheshti University of Medical Sciences Hospitals in Tehran from 2010 to 2011, based on gender, age and the level of antibiotic resistance. The use of this information can be of value to consider appropriate treatment regimens.
3. Patients and Methods
In this descriptive study, 192 patients with dyspepsia who presented clinical symptoms of abdominal pain, flatus, heart-burn, nausea and vomiting, after being examined by a gastroenterologist, underwent upper endoscopy. Informed consent was obtained and those who volunteered were asked to fill a questionnaire including age, sex, and drug history. Excluding criteria in the study were previous use of antacids (e.g. Omeprazole, Bismuth) and antibiotics. To perform an endoscopy, all of the patients were first prepared with the help of ward staff, and finally two samples of gastric antrum were biopsied by the gastroenterologist. Histological assessment: samples were fixed in 10% formaldehyde solution, then put into paraffin under the biological hood and cut into four-micrometer slices by a sterile pincet. For histological assessment and H. pylori diagnosis, hematoxylin-eosin (H & E) and Giemsa stains were used. In this study, the extent of H. pylori infection and severity of gastritis for all the samples were measured based on the latest version of Sydney system for the classification of gastritis and categorized into “mild and moderate” and “severe” (12). Moreover, association between H. pylori and severity of gastritis was assessed.
Culturing of the samples and antibiogram test to determine antibiotic resistance: biopsy slices were cultured on Brucella agar medium (Merck Co., Germany) containing 10% sheep blood, vancomycin (6 mg/L), trimethoprim (5 mg/L), and amphotericin B (2 mg/L). The cultured plates were incubated in a micro-aerobic atmosphere (5% O2, 10% CO2, and 85% N2) of a CO2 incubator (Innova Co-170; New Brunswick Scientific, Edison, NJ, USA) at 37ºC for three to five days. Grown colonies were identified using urease, catalase and oxidase tests as well as Gram staining (8, 13). Next, to determine antibiotic sensitivity, an antibiogram test was done using the modified disk diffusion method. At this stage, 100 μL bacterial suspension was as prepared equivalent to the No. 3 McFarland standard and cultured on Mueller-Hinton agar medium (Merck Co., Germany) containing 5% defibrinated sheep blood. Finally, antibiotic disks were placed on the medium and stored in a CO2 incubator in microaerophilic conditions for three to five days. Following the incubation, antimicrobial effects of antibiotics were categorized into sensitive and resistant by measuring the inhibition zone diameters (based on CLSI standards) in millimeters (14). It should be mentioned that in this study, semi-sensitive status was not considered. Antibiotic disks used were tetracycline (Patan teb.), amoxicillin (Patan teb.), metronidazole (MAST, London), ciprofloxacin (Patan teb.) and clarithromycin (MAST, London). Statistical analysis: data was analyzed using the SPSS software (version 16.0). For categorical variables the chi-square test and for continuous variables the T-test was used.
4. Results
Of the 192 patients, 85 were female (45%) and 107 were male (55%). The youngest was a 16-year-old female and the oldest was an 88-year-old female. Mean ± SD age of the patients was 48.9 ± 16.9 years. The frequency of H. pylori infection was 83%. In those younger than 20 years of age, the frequency was lower than that of the older group and despite observing more positive test results in the older age groups, this was not statistically significant (P value = 0.182) (Table 1).
| Age | Helicobacter pylori, Negative, No. (%) | Helicobacter pylori, Positive, No. (%) | Total Patients |
|---|---|---|---|
| 0 - 20 | 2 (50) | 2 (50) | 4 |
| 20 - 40 | 8 (13) | 54 (87) | 62 |
| 40 - 60 | 10 (15.2) | 56 (84.8) | 66 |
| ≥ 60 | 13 (21.6) | 47 (78.4) | 60 |
| Total | 33 (17) | 159 (83) | 192 |
Frequency and Percentage of H. pylori Infection in Patients Referred to Shahid Beheshti University of Medical Sciences Hospitals in Tehran from 2010 to 2011 (Age-Wise)
Frequency of H. pylori infection in female subjects was 80% and in males was 85.1%. Considering the severity of H. pylori infection, in the “severe” category, 35 subjects were male (71%) and 17 subjects (29%) were female (P = 0.056). However, no statistically significant correlation was found between sex and severity of infection (P = 0.466) (Table 2).
| Gender | Helicobacter pylori, Negative, No. (%) | Helicobacter pylori, Positive, No. (%) | Total Patients |
|---|---|---|---|
| Male | 16 (14.9) | 91 (85.1) | 107 |
| Female | 17 (20) | 68 (80) | 85 |
Frequency and Percentage of H. pylori Infection in Patients Referred to Shahid Beheshti University of Medical Sciences Hospitals in Tehran from 2010 to 2011 (Gender-Wise)
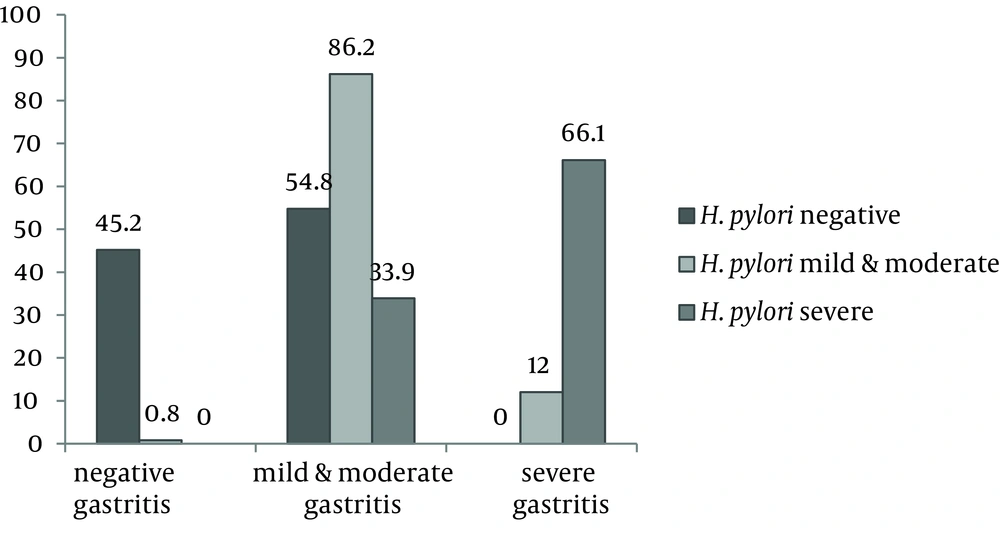
In this survey, 176 of 192 patients had gastritis. Frequency of H. pylori in these 176 cases was 90.3% (CI 95% = 85.9% - 94.6%). There was a significant correlation between severity of gastritis and H. pylori infection. Of the 53 subjects with severe H. pylori infection, 35 patients also had severe gastritis (66.1%), while among subjects with mild to moderate H. pylori infection only 13 (12%) had severe gastritis (Figure 1).
The resistance status of isolated H. pylori samples from patients were as follows: resistance rates to metronidazole, clarithromycin, amoxicillin, ciprofloxacin and tetracycline clarithromycin, amoxicillin, ciprofloxacin and tetracycline were 50%, 16.1%, 5.2%, 4.6% and 3.6%, respectively.
5. Discussion
Based on the results of this study, the frequency of H. pylori infection in our patients was 83%. This finding is in accordance with previous studies conducted in developing countries, reported that the rate of prevalence for H. pylori infection was approximately 75% , while studies in the developed countries have shown a much lower prevalence rate, which was around 40% (15-17). Considering the fact that in this study subjects had dyspepsia, a higher frequency would be expected. Although some studies have suggested an increasing rate of H. pylori infection with age (18), this study did not find such association. In this study, children were not included, so no comparison could be made with studies investigating this age group. In 2007, Sasidharan et al. showed a significantly higher frequency for H. pylori infection among males, compared to females (51.9% versus 33.1%) (19). According to many studies, differences observed between genders might be due to different life styles such as cigarette smoking and alcohol consumption, which in males can be a predisposing factor for H. pylori infection (20). Furthermore, iron deficiency anemia in women as a result of menstruation can decrease the frequency of H. pylori infection (21). In contrast, some researchers believe H. pylori infection dose not correlate with gender (22). We showed that 71% of severely infected subjects were males, while only 29% were females. However, overall analysis failed to show any significant association; infection in men was only 5% higher than women.
Like many other studies, this study showed that H. pylori infection is associated with gastritis (23, 24). We observed a significant correlation between H. pylori virulence and severity of gastritis. H. pylori had the strongest correlation with “severe gastritis”. Among patients with severe H. pylori infection, 66.1% had severe gastritis. A study in Jordan revealed that about 82% of dyspeptic patients, most of whom had gastritis, were infected with H. pylori (25). In 2003, another study showed that prevalence of H. pylori infection in patients with upper gastrointestinal disorders like gastritis was 68.5%. This result is suggestive of a strong correlation between H. pylori infection and development of gastritis in different populations (26). With an assumption that there is high prevalence of H. pylori infection in our country, high risk patients should be treated to prevent development of gastric cancer. Moreover, specific analysis to detect drug resistance must be undertaken to prevent treatment failure of H. pylori infection. In samples assessed in this study, resistance to metronidazole was considerably high (50%), which corresponds with the results of the Siavoshi et al. (55.6%) (27) and Mohammadi et al. studies from Tehran (52%) (8). It seems that widespread use of metronidazole in H. pylori and parasitic infections has resulted in the emergence of highly resistant species to this antibiotic (28, 29).
In this study, resistance rate to clarithromycin was 16.1% and this is similar to the rates reported by Rafeey et al. (30) and Mohammadi et al. (8), which were 16% and 17%, respectively. Other studies reported 7.3% (27) and 30.1% (31) as the rate of resistance to clarithromycin (27, 31). In this study, rate of resistance to amoxicillin was 5.2%, which was similar to that of one study from Tehran performed during 2010 (7.3%) (27). However, it was lower than the results of studies from Kerman (27%) and Shiraz (20.8%) (31, 32). Rate of resistance to ciprofloxacin in this study (4.6%) was similar to those reported from Tehran, during 2007 (30) and Kerman, during 2009 (31), which were 7% and 7.9%, respectively. Rate of resistance to tetracycline in this study (3.6%) was to some extent similar to other studies (31, 32). In this study, rate of resistance to furazalidone was not determined. However, regarding the limited number of studies, which have investigated resistance to this drug, an increasing rate of resistance is suggested. In a study by Siavoshi et al. in 2000 (33), this rate was 0% while in 2010 (27) it was 4.5%.
Studies worldwide, have reported different antibiotic resistance rates. Boyanova et al. in 2010 showed a high resistance rate to clarithromycin (over 20%) in the US, developed countries, Europe and Asia. The highest resistance rate to metronidazole (over 80%) was reported for Africa, Asia and South America. While primary resistance to amoxicillin was at a low level in Europe (0% to less than 2%), it was higher in Africa, Asia and South America (6% to 59%). Similarly, resistance to tetracycline in most countries was either at a low level (< 5%) or had never existed, while it was higher in Asia and South America; 9% to 27% (34). These differences may be justified by the presence of different H. pylori strains, all over the world as well as different levels of antibiotic misuse (34). In conclusion, prevalence of H. pylori infection in our patients was 83%, similar to that of developing countries. Moreover, severity of gastritis was correlated with the extent of H. pylori infection. In addition, the antibiotic resistance rate observed in this study signifies the ever-growing importance of further antibiotic sensitivity studies to help with proper treatment regimens against H. pylori.