1. Background
Leishmaniasis is a vector-borne disease, caused by obligate intracellular protozoa of the genus Leishmania and transmitted by phlebotomine sandflies Cutaneous Leishmaniasis (CL) exists in two epidemiological forms: zoonotic CL, due to L. major which provided in rural areas where the gerbil is the main reservoir host, and anthroponotic CL is caused by L. tropica in large and medium size cities, where humans are the main reservoir host (1). Cutaneous leishmaniasis is endemic in 88 countries worldwide, with more than 350 million people at risk and incidence of 1.5 - 2 million new cases annually (2). Approximately, 90% of CL cases occur in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria, Brazil, and Peru (3).
It is estimated that CL is the ninth cause of disease burden among individual infectious diseases (4). Cutaneous leishmaniasis is an important health problem in many countries of the southern and eastern Mediterranean, with diversity of clinical manifestations from simple and single lesions to extensive lesions. Despite the spontaneous healing of these lesions, the facial scars can be a social stigma, especially for women (5).
In our country, CL occurs in several old urban foci, where cases are most often observed in young children, as the older population is immune due to previous contacts with the parasite. In the district of Bam city (one of the closest towns to the south of Iran), there has been an eightfold increase in the number of cases reported over the last five years after the 2003 earthquake (6). Studies showed that the increase in CL incidence in regions is attributed to new settlements, urbanization, agricultural development, migration, improvement of reporting systems and ecological changes (7-9).
The prevalence of infection has been reported as 1.8% to 37.9% in different provinces of Iran (10, 11). However, enough knowledge about epidemiological aspects of the CL disease is needed to launch a proper program to plan control and preventive strategies about the disease.
2. Objectives
The present study was performed to determine the epidemiological aspect of CL in Iran during 6 months of 2014.
3. Patients and Methods
In this cross-sectional study, all cases of CL reported to CDC by state health departments from March 2014 to September 2014 were enrolled.
Cutaneous leishmaniasis is included in the list of mandatory notifiable diseases in Iran. In other words, private clinics, laboratories, and public centers are committed to report all of diagnosed CL cases to the department of preventive diseases. Islamic Republic of Iran is located in west of Asia with a population of about 78 million people and an area of 1,648,195 km2.
A confirmed CL case is an individual with clinical manifestations, compatible with CL (appearance of skin lesions, nodular or ulcerative, usually on exposed areas of the body, which can be followed in some cases by the appearance of mucosal lesions), and laboratory confirmation via detection of the pathogen on clinical samples (if only mucosal lesions exist, laboratory confirmation will be performed via serology).
The checklist used included demographic information, such as age, gender, occupation, a history of travel in the past year, and the travel location), clinical information (a history of previous scar, the location and size of the wound, the number of wound, the shape of wound, and the type of treatment regimen).
To analyze data, description measures such as frequency tables, measures of statistical central and dispersion values were used. All the analyses were done using STATA software version 11 (Stata Corp, College Station, Texas, USA). Additionally, area maps were created using ArcView GIS v. 3.3.
4. Results
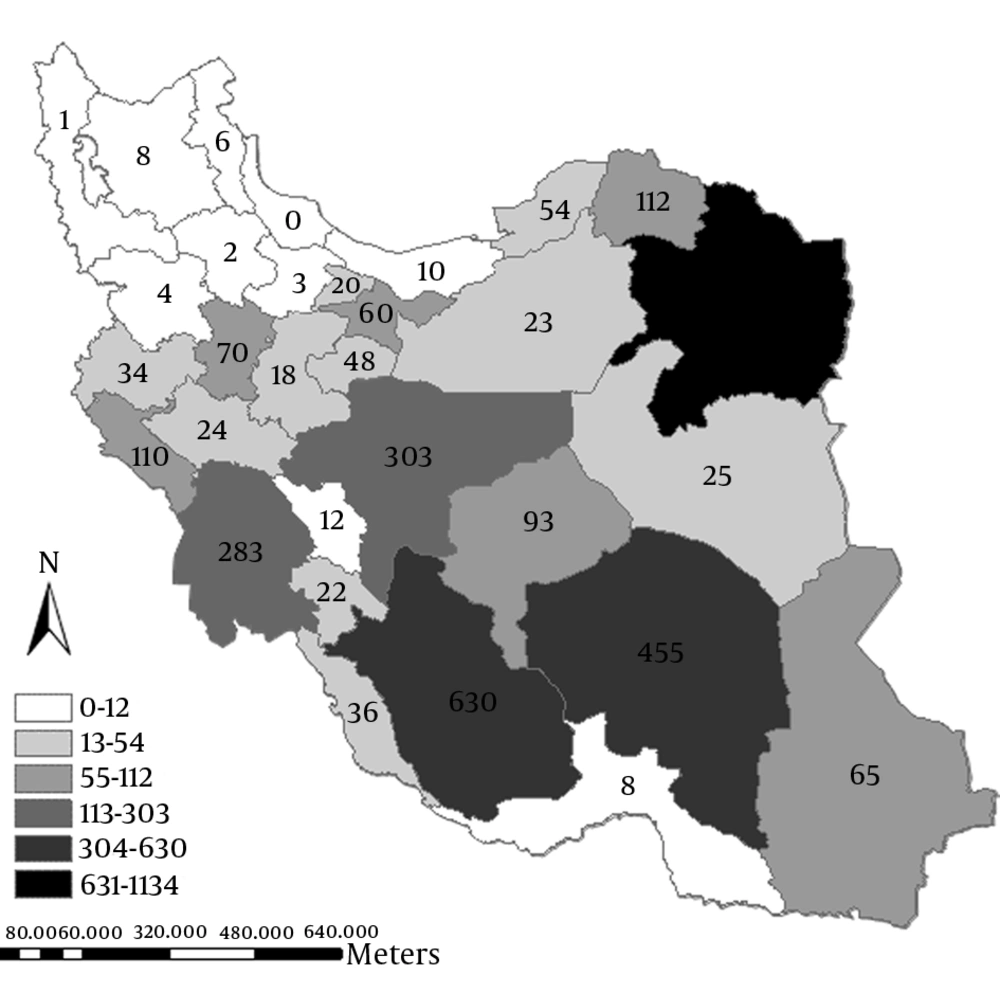
Overall, 3684 patients with CL were reported to CDC from March 2014 to September 2014 in Iran. The geographic distribution of cases according to the province is shown in Figure 1. Most of the cases were observed in eastern, central, and southern provinces in the way that the most frequent cases were in Khorasan-e-Razavi (1134 cases) Fars (630 cases), and Kerman (465 cases), while north and northwest areas had reported fewer cases.
Demographic characteristics of the study population are shown in Table 1. According to nationality, the patients classified into Iranian patients (3431, 93.14%), Afghanis (246, 6.68%), Pakistanis (4, 0.11%), and were Iraqi (3, 0.08%). Overall, 2031 cases (55.13%) were male and 2,306 (62.6%) were living in urban areas. The mean age of patients was 27 ± 18.
| Variables | Number |
|---|---|
| Gender | |
| Male | 2031 (55.13) |
| Female | 1653 (44.87) |
| Age group, y | |
| 0 - 14 | 1146 (31.11) |
| 15 - 24 | 673 (18.27) |
| 25 - 44 | 1132 (30.73) |
| 45 - 54 | 345 (9.36) |
| More than 55 | 388 (10.53) |
| Location | |
| Urban | 2306 (62.6) |
| Rural | 1352 (36.7) |
| Mobile | 26 (0.71) |
| Scar history | |
| No | 3644 (98.9) |
| Yes | 40 (1.1) |
| Nationality | |
| Iranian | 3431 (93.14) |
| Afghan | 246 (6.68) |
| Pakistan | 4 (0.11) |
| Iraq | 3 (0.08) |
| Travel history b | |
| Yes | 963 (26.14) |
| No | 2721 (73.86) |
| Referral unit | |
| Health centers | 2235 (60.67) |
| Health houses | 243 (6.6) |
| Private centers | 781 (21.2) |
| Hospitals | 86 (2.3) |
| By self | 18 (0.5) |
| Others | 321 (8.7) |
a Data are presented as No. (%).
b Travel history in last year.
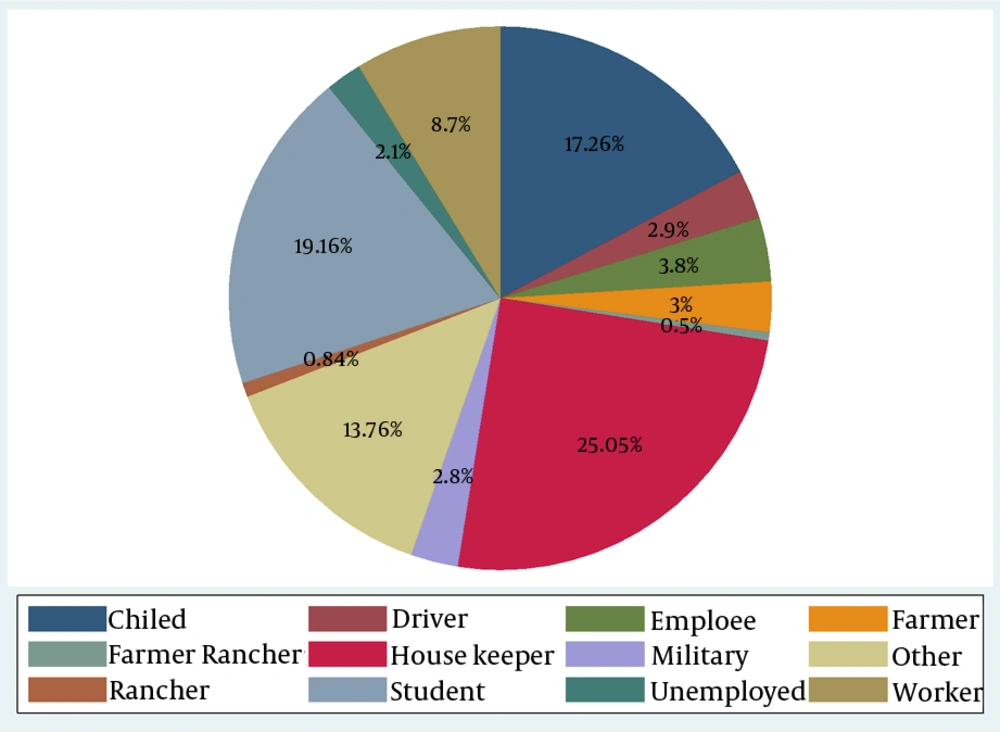
In addition, more than 31% of the participants were under 14 years of age while 10.53% of them were found to be over 55 years. In this study, 2235 subjects (60.67%) were related to health centers, 2.3% and 21.2% for hospitals and the private sector, respectively. Only 963 (26.14) of the patients reported a history of travel in the past year and 1.1% of them had a history of previous scar. Occupational status of the patients is shown in Figure 2. Housewives had the highest incidence (25.05%), following by students and children (incidence rate: 19.16% - 12.76%, respectively).
Clinical characteristics of the study population are shown in Table 2. Among study subjects, 3570 individuals (96.91%) were new cases. Treatment failure was reported in less than 1% of the patients. More wounds were seen in open and uncovered organs of the body such that 62.75% of wounds were seen on the hands, 24.8% in the head and neck, and 2.7% in other parts of the body. In over half of the cases (53.18%), wound size was one or less than one centimeter but about 6% of the wound were more than 4 centimeters. The shape of ulcer wound without purulent discharge was seen in 2472 patients (67.1%) and there were about 32% of cases with ulcers and purulent discharge. Only one wound was observed in 53.23% of the cases while there were about 16.4% with more than three ulcers in the organs. Nearly, all of the cases (97%) reported any underlying disease.
| Variables | Frequency |
|---|---|
| Disease type | |
| New case | 3570 (96.91) |
| Failure | 32 (0.87) |
| Recurrence | 57 (1.54) |
| Other | 25 (0.67) |
| Lesion Location | |
| Hand | 2312 (62.75) |
| Face and neck | 915 (24.8) |
| Body | 98 (2.7) |
| Foot | 837 (22.7) |
| Lesion size, cm | |
| 1 | 1959 (53.18) |
| 2 | 923 (25.05) |
| 3 | 427 (11.59) |
| 4 | 152 (4.13) |
| More than 4 | 223 (6.05) |
| Lesion shape | |
| Ulcer and secretions | 1177 (31.95) |
| Ulcer | 2472 (67.1) |
| Lupoid | 26 (0.71) |
| Osporotrikoid | 9 (0.24) |
| Lesion number | |
| 1 | 1961 (53.23) |
| 2 | 769 (20.87) |
| 3 | 350 (9.5) |
| More than 3 | 604 (16.4) |
| Underlying disease | |
| No | 3584 (97.29) |
| Diabetes | 33 (0.9) |
| Hypertension | 54 (1.4) |
| Liver disease | 5 (0.13) |
| Other | 15 (0.41) |
a Data are presented as No. (%).
5. Discussion
Currently, zoonotic diseases (Zoonosis) are one of the major public health challenges, and due to expanding urbanization, migration types, the speed of deforestation, along with other environmental actions, are considered important health problems (12-15). The CL is one of the major health problems in the Middle East, including Iran and the Mediterranean littoral (16). According to a report submitted to the world health organization in 2008, Iran had the highest prevalence of the disease in the region, with 26,869 new cases which more than 67 percent of them were rural residents (17). Notwithstanding, in the 6-month study period, more than 62% of cases were urban residents indicating a change in the epidemiological pattern in the country. It is noteworthy that in the foci of rural zoonotic CL, seasonal distribution of the disease usually begins from September and reaches its peak in December. Therefore, considering duration of the study, it is expected that rural cases are more than what have been found throughout the year. High frequency of CL in Iran can be investigated from two aspects. First, the high frequency could be due to the border with three countries: Iraq, Afghanistan, and Pakistan. As also shown in this study, nearly 7% of those detected in the country in the period of 6 months were immigrants from the aforementioned countries. Because of illegal immigration to the country by many Afghan nationals, living in high risk areas, and lack of adequate access to health services, underestimated rates are likely expected. Second, there is greater sensitivity of the surveillance system in detection and treatment of the disease in the country compared with other countries in the region. However, many experts believe that there is undercount or underestimation of the disease due to lack of awareness of the symptoms of the disease (16). Our findings, similar to other studies, showed that tropical regions in Iran like Khorasan, Fars, and Kerman provinces had the highest incidence of the disease consistently (18, 19). Other studies expressed that the effect of temperature and climatic conditions is known as a risk factor for the life cycle of the carrier, the frequency in animal reservoirs, and the transmission pattern of the predominant type pathogen (20-22). It is somewhat difficult to compare the distribution of age groups according to different designs and classification in various studies (23). Our study indicated that age group of o to 14 years had the highest rates, which is similar to studies performed in Turkey and Greece (20, 24) while in a study conducted in Brazil the lowest prevalence was seen in this age group (25). Given that infection with Leishmania provides immunity against reinfectin with the same parasite, most children will be infected in endemic areas. It is important to pay more attention to undercount of CL in this age group because of impetigo and folliculitis (26). The age pattern of CL indicates that the disease is commonly seen in children less than ten years; and in areas with lower prevalence, involves adolescences and young people, in addition to children. Our results showed that older age groups also were infected. This may be due to infection in areas where the disease recently occurred. Clinical symptoms of Leishmania parasite, especially major Leishmania, vary in different regions of the world, depending on the type of parasite as nodular, ulcerative, satellite lesions, lymphadenitis, and sporotrichoid (27, 28) In this study, consistent with findings obtained from endemic countries, 67% of the subjects had ulcer (25, 29). It has been documented that any painless lesions in endemic regions last more than two weeks should be considered suspicious for CL (16). The location of wound in various regions is also a function of type of mosquito’s activity, culture of wearing clothing, and exposure to carrier. In the present study, approximately 87% of the wounds exist on the hands, face, and neck because of sleeping in the outdoor, without the use of linen and lace, and lack of proper body cover in summer, considering the high prevalence of the disease in tropical regions, while in Brazil more than 34% of wounds occurred in the legs, given the type of wearing clothing among Brazilians (25). Other studies have also shown a higher frequency of the disease in men (23, 30). In our study, 55% of the cases were men. This may be due to higher exposure to mosquito’s bites and business trips to endemic areas. In a 10-year study in England, 71% of patients admitted to hospital had a history of travel to endemic regions, especially the Mediterranean countries (31). A study in Afghanistan on Dutch soldiers indicated the prevalence of 18.3% among them because they are susceptible at the time of migration (29). Further studies are needed to determine the role of sex and gender susceptibility. Since 46.7% of patients had two or more ulcers, we can conclude that some types of Phlebotomus bite more than once a host based on physiological characteristics and feeding habits, and in each bite Leishmania parasites are injected into the blood. It is necessary to identify different types of carriers and their feeding habits in endemic areas to prevent further ulcers (26).
Finally, the main limitation of this study is the short period of this study. Hence, international comparisons are somehow controversial. It is suggested that trend of the disease is evaluated in further studies with a longer period of time. It is also advisable to conduct more analytical studies to determine the role of socioeconomic factors such as poverty, development, human behavior, protein-energy malnutrition, population dynamics, entering nonimmune individuals into inside.
Most patients in this study were from eastern and southern regions of the country with warm weather and dry climate. The greater involvement of patients in the age group 0 to 14 years and prevalence of 25% in housewives revealed that the vector exists indoor and outdoor places. Therefore, protection and prevention actions should be more aggressively pursued near homes, especially in endemic areas. Open parts of the body, such as the head and face, had more lesion involvement. As a result, more training is required especially in tropical regions and the use of topical ointment to protect open organs. According to the epidemiological features of CL in Iran, providing a uniform mechanism for control and prevention of this disease is not possible.