1. Background
Bacterial infections are the cause of significant mortality and morbidity in children. Urinary tract infection (UTI) is one of the common infections in pediatric wards, worldwide (1, 2). Urinary tract infection can be nosocomial or community acquired (3). It is defined as the growth of > 105 CFU/mL of a single pathogen from properly collected urine specimen of children with febrile disease or UTI symptoms (4). The infection may involve only lower or both upper and lower parts of the urogenital system (5). The rate of UTI usually depends on age and sex. The incidence of UTI is greater in girls as compared to boys, which may be either due to anatomical structure or physiologic mechanisms (6, 7). In boys, UTIs are much more common in uncircumcised boys especially during the first year of their life (8). Enteric Gram-negative bacteria are the most common cause of urinary tract infections, with Escherichia coli being the predominant pathogen (5, 6). Gram-positive bacteria, including Staphylococcus spp and Streptococcus spp (e.g. S. saprophyticus, Enterococci) have also been reported as pathogens in children with UTI (8-10). Delay in diagnosis and appropriate treatment is the cause of complication in the upper genitourinary system (5). There has been an increase in the rate of antibiotic resistant bacteria associated with UTI in pediatric wards (4, 11, 12). Also exchange of antibiotic resistance genes among the uropathogenes causes multi-drug resistance to antibiotics (13). Effective antimicrobial therapy for UTI is important and can reduce adverse effects in these patients.
Therefore, there is growing concern regarding the resistance of urinary pathogens to antimicrobial agents because of the increasing number of therapeutic failure after empiric treatment. Most recent studies, particularly in developing countries, on the antimicrobial aspect of bacterial pathogens causing UTI in children, have shown high levels of antibiotic resistance in clinical and community settings (4). Awareness of the antimicrobial resistance patterns of common uropathogens in children, according to local epidemiology, is essential for providing clinically appropriate, cost effective therapies for UTIs. To the best of our knowledge, there is a lack of available information on bacterial agents and their antibiotic susceptibility pattern regarding pediatric UTIs in Shiraz, Iran. Surveillance activities are the first step in developing infection control programs and may help decrease incidence.
2. Objectives
The aim of this study was to investigate common uropathogenes and their antibiotic susceptibility patterns in the general pediatric ward of Dastgheib hospital in Shiraz, the southwest of Iran.
3. Patients and Methods
This cross-sectional descriptive study was conducted from March 2009 to March 2011 on 2854 children (69.1% girls), admitted with UTI to the pediatric ward of Dastgheib hospital, a general teaching and therapeutic hospital with seven wards and 133 beds, stablished in 1950. It is a major pediatric referral center with about 80,000 annual admissions. Patients’ information, including age, gender, clinical status and the type and number of isolated bacteria, were recorded in a designed form. The study subjects were divided to three groups: 43.4% neonates (one month to one year), 38.1% infants (one to five years) and 19.5% children (> 5 years). This study only included cases, who had not received antibiotic treatment before hospitalization. Subjects with incomplete medical report and no diagnostic antibiogram test were omitted. This study was approved by the ethics committee of Shiraz University of Medical Sciences. The ethics committee waived the need for informed consent because we used medical records. It did not harm any of the patients. The patients’ personal details were kept strictly secure and confidential.
3.1. Microbiological Methods and Antibiotic Susceptibility Testing
Samples were collected in a sterile container and sent to the laboratory by midstream, urine bag, catheter and suprapubic methods based on age and physical status of the subjects. Five percent blood agar, eosin methylene blue and MacConkey agar (Himedia, India) were used for culture. Bacterial identification was done using conventional methods. Positive results were considered if the number of colonies of a single organism were more than 100,000 CFU. Mixed growth of bacteria was considered as contamination.
Antimicrobial susceptibility testing was performed by disk diffusion methods, as recommended by the clinical laboratory standard institute (CLSI) on the most prevalent Gram-negative and positive isolated bacteria. The following antibiotic disks (Mast, UK) were used: imipenem (10 μg), ciprofloxacin (5 μg), nitrofurantoin (300 μg), gentamycin (30 μg), ceftriaxone (30 μg), cephalexin (30 μg), cefixime (5 μg), co-trimoxazole (1.25/23.75 μg), nalidixic acid (30 μg), cefotaxime (30 μg), vancomycin (30 μg), ampicillin (10 μg) and penicillin (10 units). Standard strains used for the susceptibility tests were Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213 and Pseudomonas aeruginosa ATCC 27853. The isolates were classified as sensitive or resistant according to CLSI guidelines.
3.2. Statistical Analysis
The SPSS software (version 15) was used to address the frequencies and descriptive analyses.
4. Results
From all urine cultures, 460 (16.1%) samples were positive and 2394 (83.9) were negative. Out of positive urine cultures, 310 samples were related to girls (67.4%) and 150 to boys (32.6%). The most infected group was infants aged between one and five years (4.6 ± 3). In total, UTI prevalence was more in girls as compared to boys. In positive urine samples, Gram-negative bacteria were more than Gram positive ones.
Escherichia coli with 300 cases followed by Klebsiella with 50 cases were the most prevalent Gram-negative bacteria and Staphylococcus saprophyticus with 50 cases was the most prevalent Gram-positive bacteria (Table 1).
| Type of Isolated Bacteria | Number of Bacteria, No. (%) |
|---|---|
| E. coli | 300 (65.2) |
| S. saprophyticus | 50 (10.9) |
| Streptococcus spp. | 30 (6.5) |
| Klebsiella spp. | 50 (10.9) |
| Enterobacter spp. | 20 (4.3) |
| Pseudomonas spp. | 10 (2.2) |
Distribution of Bacterial Agents Isolated From Urine Specimens
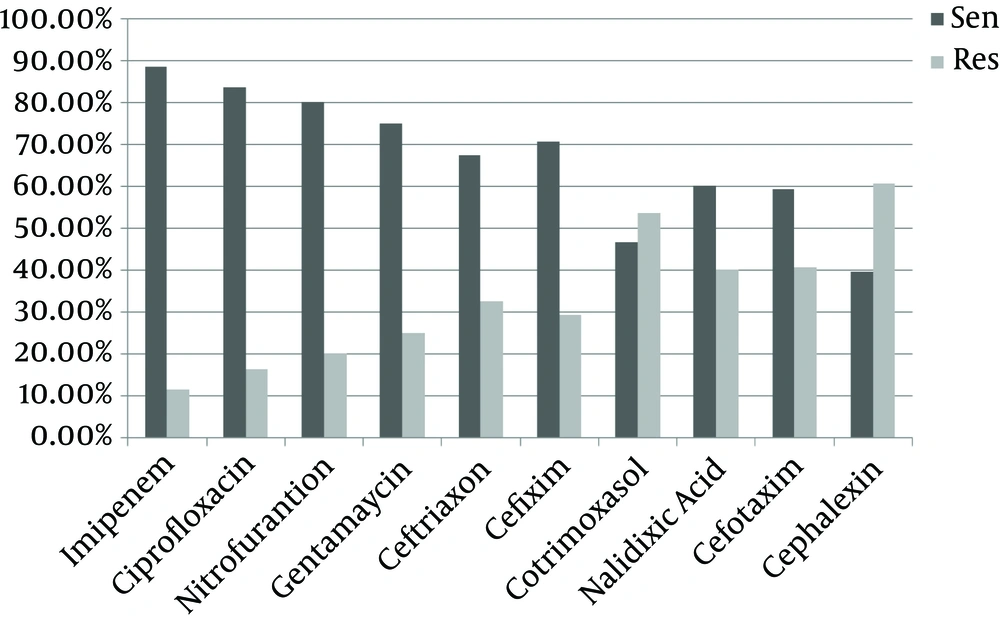
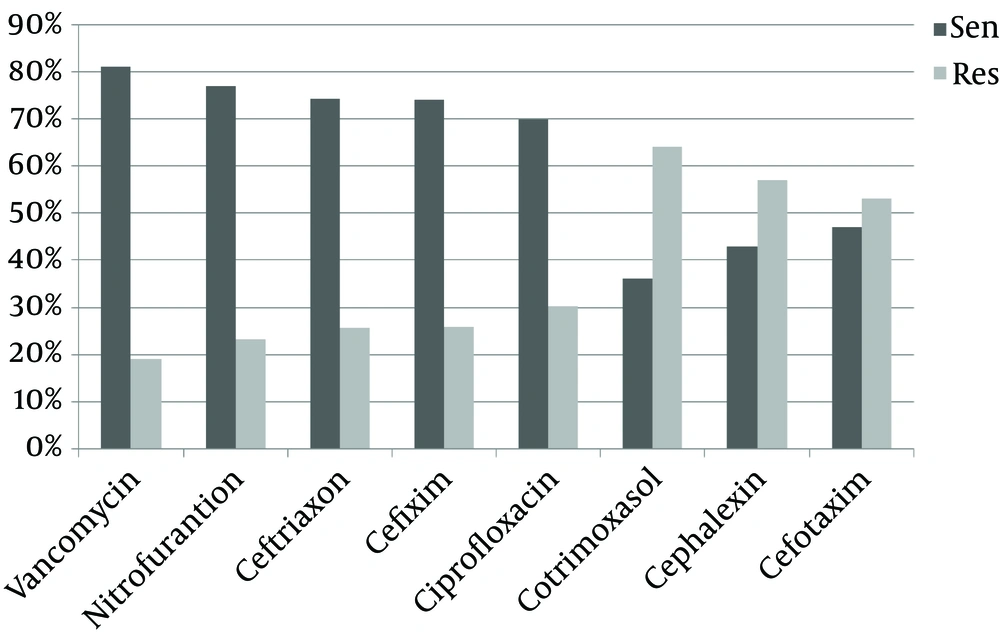
Antibiotic sensitivity of E. coli and Staphylococcus saprophyticus is shown in Figures 1 and 2. Escherichia coli showed more sensitivity to imipenem (88.4%) and ciprofloxacin (83.7%) and resistance to cefalexin (60.5%) and co-trimoxazole (53.5%). Also S. saprophyticus showed more sensitivity to vancomycin (81%) and nitrofurantoin (76.8%) and resistance to co-trimoxazole (64%) and cephalexin (57.1%).
5. Discussion
Urinary tract infection (UTI) is one of the prevalent infections in pediatric wards all around the world; however, its etiologic agents have changed during the recent decade. Our data was obtained from patients of a hospital in Shiraz; therefore this study does not indicate the general results for all patients in Iran.
Urinary tract infection ratio is quite variable between hospital pediatric wards or laboratories of different cities in Iran. For example, in Tabriz (central laboratory of Tabriz University of Medical Sciences, age group between 1.5 to 65 years old), Qazvin (Qoda pediatric hospital, age group between three months up to 12 years old) and Bandar Abbas (Children’s hospital, age group one week to 16 years) the UTI ratios were 13.2%, 7.2% and 7.87%, respectively (14-16), compared with the present study with UTI ratio of 16.1%. Based on different studies, UTI is more prevalent in girls (3% - 5%) than boys (1%) (17, 18). In this study from 460 positive urine cultures, 67.4% belonged to girls. Because the majority of reports were the same, we can conclude that short urethra, anatomic position of urinary system and rapid bacterial transfer to the upper part of the urinary system increases the risk of UTI in girls (4, 5).
Escherichia coli and S. saprophyticus were the most frequent Gram negative and positive isolated bacteria in the order of 65.2% and 10.9%, respectively in the present study. Likewise, many scientists reported E. coli as common pathogenic isolates in UTI (12, 19-21); however, in some geographic regions such as Nigeria (Ebonyi State university teaching hospital, Children’s emergency unit, age group between birth to five years old) Klebsiella spp was reported as the main factor of UTI in children (22). In Iran, Monsef and Eghbalian and Mirsoleymani et al. reported Klebsiella spp as the second factor for UTI besides E. coli (Ekbatan hospital of the Hamadan University of Medical Sciences, neonatal and neonatal intensive care unit, age group between one to 30 days), as shown in our study (11, 16). Furthermore, some scientists indicated that Klebsiella spp is more isolated from boys with UTIs; it is hypothesized that etiological agents of urinary tract infection vary between both genders (23).
Similar to reports by Afsharpaiman et al. (Najmieh hospital of Tehran, pediatric ward, age group between < 28 days to > 2 years) and Farshad et al. (Motahary hospital of Jahrom, age group between one month to 14 years old), E. coli isolates in this study showed a high sensitivity to Imipenem; this confirms the sensitivity of these bacteria to carbapenems (12, 19). However, in another study nitrofurantoin and ceftriaxone were the most effective agents against E. coli in Iran (central diagnostic lab of Karaj) (4, 5, 24). In Nigeria, gentamicin was the most effective antibiotic against E. coli (22). Different antibiotic patterns in Iran or around the world might be due to various antibiotic prescriptions in diverse geographic areas, broad-spectrum antibiotic therapy for UTI and outbreak of antibiotic differences in pathogenic bacteria by mutation. Cephalexin and co-trimoxazole showed a higher resistance pattern to these bacteria. In other studies conducted in different parts of Iran, E. coli showed resistance to co-trimoxazole, ampicillin and cephalexin (5, 12, 19, 24). This could probably be due to the overuse of these antibiotics in the whole country, which has resulted in bacterial resistance.
Based on Monsef and Eghbalian research, S. saprophyticus was the most Gram-positive isolated bacteria in the urine culture; this is similar to the results of the present research (11). However, the antibiotic susceptibility pattern was opposite so that in our research vancomycin and nitrofurantoin were the most effective antibiotics, and co-trimoxazole was the less effective antibiotic for S. saprophyticus while in the study of Monsef and Eghbalian these bacteria were sensitive to co-trimoxazole and resistant to vancomycin. However, S. saprophyticus was resistant to cephalexin in both studies (11).
The review of the patients’ information showed that the most common antibiotic, which was prescribed by physicians for hospitalized children with UTI before obtaining a culture result, was ceftriaxone. This antibiotic was different from most effective antibiotics found in the present study (imipenem). This shows the use of inappropriate treatments for UTIs, which may increase bacterial resistance to such antibiotic.
A weakness of our study was its failure to provide separate data for symptomatic versus asymptomatic patients, as significant bacteriuria may imply that symptomatic patients were included.
5.1. Conclusion
It is necessary to prescribe antibiotics under an exact surveillance in teaching hospitals, so effective antibiotics can control infections. On the other hand, they can prevent unnecessary expenditure for using inappropriate antibiotics, which may saturate hospitals with such antibiotics causing emergence of antibiotic resistant bacteria.