1. Background
The endogenous vaginal microbiota plays an important role in preventing genital and urinary tract infections in females. Thus, an accurate understanding of the composition and ecology of the ecosystem is important to understand the etiology of such diseases (1-3).
Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum are fastidious bacteria belonging to Mollicutes class. Lack of a cell wall, coupled with their extremely small genome and limited biosynthetic capabilities, explain the parasitic or saprophytic existence of these organisms, their sensitivity to environmental conditions and fastidious growth requirements (4). There are several human pathogens in the genera Mycoplasma and Ureaplasma, responsible for a variety of clinical manifestations involving multiple body systems (5, 6).
Mycoplasma is a venereal and vertically transmitted infection. Approximately 21% - 53% of asymptomatic sexually active females are colonized with this microorganism in the cervix or vagina, while the occurrence is somewhat lower in the male urethra, being M. hominis frequently recovered (7-9). This specie is associated with systemic infections and a variety of conditions, including pyelonephritis, pelvic inflammatory diseases, chorioamnionitis, postpartum endometritis bacterial vaginosis, arthritis, osteoarthritis, wound infections and several conditions in neonates (e.g. congenital pneumonia, meningitis, bacteremia and abscesses) (4).
Ureaplasma spp. are currently composed of two important species in humans: Ureaplasma urealyticum and Ureaplasma parvum, both thought to be genital microbiota (10-12).
Ureaplasma parvum is more common than U. urealyticum as a colonizer of the male and female urogenital tracts and in the neonatal respiratory tract (8) however; U. urealyticum may be more pathogenic (4). They are commonly found in healthy people (40% to 80% in adults); therefore, their pathogenic role can be difficult to prove in a small population. Meanwhile, several studies reported that Ureaplasma are associated with some diseases including non-gonococcal urethritis, pregnancy complications and prenatal infections, more often than are indigenous microbiota (4, 12-14).
Although M. hominis and Ureaplasma species can be cultured, this requires technical skill to interpret microscopic colonies and takes two to five days. Molecular techniques are gradually replacing culture in the gynecological screening for sexually transmitted diseases. Real-time polymerase chain reaction (qPCR) detection of these microorganisms from clinical samples circumvents technical issues related to culture and shortens turnaround time for detection and identification (6), while it can be performed at the same clinical sample collected for liquid-based cytology, avoiding another gynecological examination for the patients. Collecting material for culture usually requires that the patient goes to a laboratory´s facility in order to perform a proper sampling. The higher sensitivity of these tests is their main advantage over tissue culture and other non-culture-based tests (e.g. enzyme immunoassay and DNA probes) (15, 16).
2. Objectives
The current study aimed to verify the prevalence of M. hominis and Ureaplasma species (U. urealyticum and U. parvum) by real time PCR in routine gynecological investigation.
3. Patients and Methods
3.1. Cross Sectional Study
The study used simple random sampling method (every member of the population under study has an equal chance of being selected). A total of 6,810 patients aged 11 to 80 years (mean age ± 35 years) who visited the gynecologist mainly for routine gynecological screening to investigate the presence of Mycoplasma hominis and/or Ureaplasma spp. from January 2015 to June 2015, in spite of pregnancy or presence/absence of genital diseases symptoms were included in the study. Exclusion criteria were: antibiotic therapy or vaginal medication three months prior to sample collection and sexual intercourse 72 hours preceding examination. All these aids were taken to avoid false negative results. The samples were analyzed at SalomãoZoppi Diagnostics, a private clinical diagnostic laboratory and a reference for gynecologists and female´s health at Sao Paulo city, Brazil. The study was performed according to good clinical practice and Declaration of Helsinki, and consent was obtained from each patient.
3.2. Cervicovaginal Samples
Specimens were collected by scrapping the ecto/endocervix and vaginal walls of each female with a sterile brush/spatula. The samples were immediately immersed and ressuspended in Preservcyt Solution (ThinPrep™ Pap Test, Hologic, Inc. USA).
3.3. DNA Extraction
Samples were homogenized and 200 µL of each transferred to a MagNA Pure LC sample cartridge (Roche Applied Science, Indianapolis, USA). DNA extraction was performed on the MagNA Pure LC 2.0 using the MagNA Pure LC total nucleic acid isolation kit (Roche Applied Science) with a final volume of 100 µL.
3.4. Lightcycler Assay
Primers and probes were designed for amplification of a 129 bp product of the gap gene of M. hominis (LightMix kit Mycoplasma hominis EC-TIB MolBiol GmbH, Germany); and a 187 bp fragment of 16S rRNA gene was used to detect but not differentiate Ureaplasma urealyticum and Ureaplasma parvum (LightMix kit Ureaplasma urealyticum/parvum EC-TIB MolBiol GmbH, Germany). Controls for monitoring the quality of DNA extraction (internal control), and the success of PCR reaction (positive and no template control-NTC) were part of the commercial kits and were included in each run. DNA amplifications were performed following the manufacturer’s instructions. The LightCycler 480 II Real Time PCR System (Roche Diagnostics, USA) was used to amplify the genes; PCR program was composed by: Hotstart Taq DNA polymerase activation done in 95°C for 10 minutes, followed by cycling: 95°C (20°C/s) for 5 seconds, 62°C (20°C/s) for 5 seconds and 72°C (20°C/s) for 15 seconds, repeated 50 times. Melting assay ended the analysis: samples were heated up to 95°C (20°C/s) for 20 seconds, cooled to 40°C (20°C/s) hold for 20 seconds and then heated slowly at 0.2°C/s up to 85°C, finally cooled to 40°C (20°C/s) for 30 seconds.
3.5. Statistical Analysis
The clinical parameters were analyzed by nonparametric Chi-square tests (it required random sampling and independence of observation). Possible correlations between M. hominis and Ureaplasma spp. and age were evaluated by the Spearman test (r). For all statistical analyses, significance levels of 5% were obtained with the GraphPad Prism version 6.0 for Windows (GraphPad Software, La Jolla California, USA) as statistical package. The sample size formula was error: 3%, confidence level: 95%, population: 6,810, sample size needed: 923 and expected power: 1.0.
4. Results
Of the 6,810 collected specimens, 2,304 (33.83%) were positive for pathogens. A total of 79 (3.42%) and 2,026 (87.93%) samples were positive for M. hominis and Ureaplasma spp., respectively; while 199 tested samples were positive for both pathogens, indicating a rate of co-colonization of 8.63% (199/2,304) (Table 1).
| Age Groups | Patients, No. (%) | Prevalencea, No. (%) | Positive Rate (%) | Mycoplasma hominis Positive | Ureaplasma spp. Positive | Mycoplasma spp. + Ureaplasma spp. Positive | P Valueb |
|---|---|---|---|---|---|---|---|
| 11 - 20 | 331 | 160 (6.95) | (48.34) | 2 | 129 | 29 | < 0.0001 |
| 21 - 30 | 2030 | 865 (37.54) | (42.61) | 33 | 762 | 70 | < 0.0001 |
| 31 - 40 | 2609 | 788 (34.20) | (30.20) | 23 | 702 | 63 | < 0.0001 |
| 41 - 50 | 1197 | 355 (15.40) | (29.65) | 16 | 316 | 23 | < 0.0001 |
| 51 - 60 | 507 | 119 (5.16) | (23.47) | 4 | 103 | 12 | < 0.0001 |
| 61 - 70 | 111 | 14 (0.61) | (12.61) | 0 | 12 | 2 | < 0.0001 |
| 71 - 80 | 25 | 3 (0.14) | (12) | 1 | 2 | 0 | NDc |
| Total | 6810 (100) | 2304 (100) | (33.83) | 79 (3.42) | 2026 (87.93) | 199 (8.63) |
Distribution of Patients According to Age Group and Detected Microorganisms
The mean age of all patients was 35.43 years (range 11 - 80 years); the average age of patients with positive samples and patients with M. hominis and Ureaplasma spp. co-colonization were 35.43 and 35.41, respectively. When the studied population was divided by age group by 10-year intervals, it was observed that most of the patients (5,836) tested by real-time PCR were 21 to 50 years old; the age groups of 11 - 20 and 21 - 30 years presented the highest positive rates (48.34% and 42.61%, respectively; P < 0.0001); whereas, the age groups of 21 - 30 and 31 - 40 years had the highest number of positive patients (865 and 788, respectively; P < 0.0001). The highest number of co-colonization by M. hominis and Ureaplasma spp. were also detected in the age groups of 21 - 30 and 31 - 40 years (Table 1).
Analysis of monthly incidence of M. hominis and Ureaplasma spp. revealed no statistically significant association between positive results and the month of sample collection (January-June) (Table 2).
| Period | No. Total of Patients | Mycoplasma hominis Positive, No. (%) | Ureaplasma spp. Positive, No. (%) | Mycoplasma spp. + Ureaplasma spp. Positive, No. (%) |
|---|---|---|---|---|
| Jan | 239 | 3 (1.25) | 85 (35.56) | 10 (4.18) |
| Feb | 1102 | 9 (0.81) | 313 (28.40) | 31 (2.81) |
| Mar | 1372 | 20 (1.45) | 411 (29.95) | 42 (3.06) |
| Apr | 1374 | 16 (1.16) | 408 (29.69) | 40 (2.91) |
| May | 1405 | 14 (0.99) | 405 (28.82) | 42 (2.98) |
| Jun | 1318 | 17 (1.28) | 404 (30.65) | 34 (2.57) |
| Total | 6810 | 79 (1.16) | 2026 (29.75) | 199 (2.92) |
Monthly Microorganisms Ratea
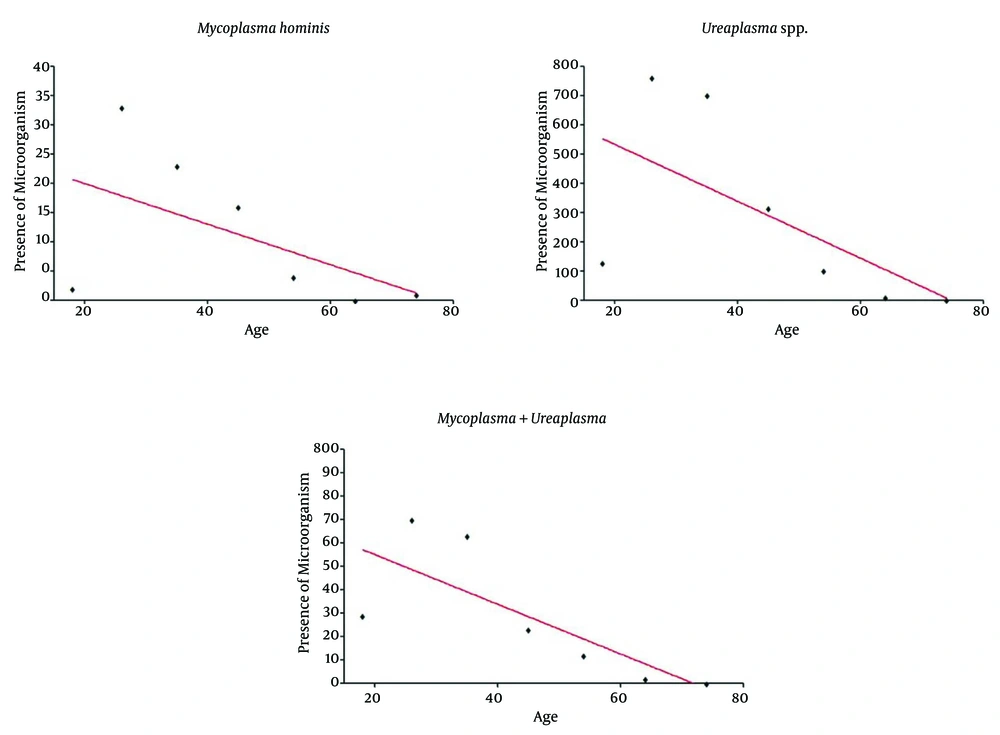
The Spearman test indicated an inverse correlation between bacterial presences detect by real time PCR and age of the females tested for M. hominis and Ureaplasma spp. (Figure 1). A negative and significant correlation was observed between the low presence of Ureaplasma spp. (r = -0.78; P = 0.048) and Mycoplasma spp. + Ureaplasma spp. (r = -0.89; P = 0.012) with the increased age of females (Figure 1B and 1C).
5. Discussion
Mycoplasma and Ureaplasma species are frequently found in urogenital microbiota of sexually active healthy females. Their colonization rates are around 80% and 40%, respectively (17-19); however, if the loads reach 104 cfu/mL, it can be a crucial criterion for urogenital infections in females. In the case of extragenital specimens in adults or neonates, a positive PCR assay or culture result should be considered clinically significant (4).
Moreover, both microorganisms are usually associated with young age, lower socioeconomic status, sexual activity with multiple partners, black ethnic groups, smoking, vaginal douching and oral contraceptive use (20-22). However, differences in prevalence according to race and socioeconomic status are reported (22-24), and differences by gender are also suggested (24).
The current study showed a high prevalence of M. hominis and Ureaplasma spp. in gynecological screening, mainly in sexually active females. The results of the present study also indicated that the prevalence of microorganisms was homogenous in each month during the study. In addition, a negative and significant correlation was observed between co-colonization of Ureaplasma spp. and M. hominis, Ureaplasma spp. and age; suggesting that the presence of these microorganisms may not be associated with increased age. This finding matches with those of the previous studies conducted in a cohort of females with bacterial vaginosis (BV) or asymptomatics (6, 22, 25), in which Ureaplasma spp. was detected significantly more often than M. hominis.
Despite more sensitivity of PCR to detect Mycoplasma and Ureaplasma species, culture remains the gold standard method, besides the most economical and practical means to detect these microorganisms especially in laboratories with a low to moderate test volume (6).
Cultures also have additional advantages because they provide isolates on which antimicrobial susceptibility testing can be performed (4). However, colonial identification is challenging and subjective, because it depends on the human eye ability and expertise. Moreover, the majority of Mycoplasma spp. and Ureaplasma spp. infection can be treated with the usual prescribed antibiotics (4, 6) there is a trend to replace this method by molecular techniques.
In a laboratory where technologists are familiar with PCR, this approach is more user-friendly (and commonly employed to detect various microorganisms) than culture (6). PCR allows a shorter turnaround time to detect these microorganisms from cytology sample used in routine gynecological examination; especially in laboratories handling a daily large volume of samples.
Molecular studies show that vaginal microbiota vary in species composition (26, 27), and therefore it is likely that they respond differently in the BV or extrinsic disturbances such as during menstruation, sexual activity or female hygiene practices among other events (28).
BV is a gynecological condition of unknown etiology, characterized by a relatively low presence of Lactobacillus spp., accompanied by a gradual change and eventually, total replacement by polymicrobial anaerobic bacteria. Among these, Prevotella spp., Mobiluncus spp., Bacteroides spp., Peptostreptococcus spp., Gardnerella vaginalis, with other bacteria including, Mycoplasma and Ureaplasma species are observed (29, 30).
BV is the cause of considerable morbidity and is the most cited cause of vaginal symptoms prompting females to seek primary health care (28, 31). Furthermore, it is reported that BV is also associated with poor pregnancy outcomes such as preterm delivery (30, 32, 33).
In pregnant females, the presence of Mycoplasma and Ureaplasma species can predispose conditions such as chorioamnionitis, spontaneous abortion, postpartum endometritis, preterm delivery and low birth weight infants (18, 34); moreover, it is also linked to female infertility (35-37).
In conclusion, the study described the prevalence of M. hominis (3.42%), Ureaplasma spp. (87.93%) and the co-colonization of both (8.63%) in gynecological samples obtained from a subset of Sao Paulo city female population submitted to routine gynecological examination. While these findings demonstrate the applicability of molecular techniques to diagnose genital infections, especially in asymptomatic females, during the second and third trimesters of pregnancy and under clinical investigation for infertility causes, they also can be considered as a pathogenic load of these microorganisms. Since most sexually active females are colonized by Mycoplasma hominis and Ureaplasma spp., limitations in highly sensitive molecular tests, such as real time PCR, should take into account the clinical relevant concentration of the microorganisms in the genital microbiota.