1. Background
Fascioliasis is one of the most important parasitic zoonosis causing public health problems in human, and economically the main disease and infection of domestic livestock especially by the reduction in productivity due to liver disorder (1). Two different reports estimated the rate of global human fascioliasis as 2.4 to 17 million, whereas the population at risk was estimated 180 million (2).
Fascioliasis emerged as a main health problem in Guilan province, North of Iran. This province experienced 2 outbreaks of human fascioliasis in 1987 and 1997, affecting more than several thousands of people (3). Moreover, many cases of human fascioliasis were reported from other provinces of Iran (4). Animal fascioliasis is quite frequent in grazing animals generally in most areas of the state and the prevalence reaches about 50% in certain provinces (5).
Morphological criteria such as body size and shape are among the traditional and important methods to distinguish between the 2 species, but these methods are not commonly trusted because of the variable range in different species (6).
The species of Fasciola are meiotically functional diploid and can produce sperm and temporarily store the produced sperms in the seminal vesicles named spermic fluke. This male reproductive organ is the common predominant characteristic of both species (7). On the other hand, intermediate Fasciola that have morphological characteristics intermediate between F. hepatica and F. gigantica with no sperm (aspermic fluke) in their seminal vesicles are found in Asian countries (8, 9).
One of the main problems in anthelmintic resistance of this parasite is due to aspects of biology and population structure and pathogenicity, and the control of fascioliasis depends on genetic diversity (10, 11). Both of these species may be identified by applying DNA sequences of nuclear ribosomal DNA internal transcribed spacers (ITS), and sequences of CO1 and ND1 as mitochondrial genes to analyze intraspecific phylogenetic relationship of Fasciola spp. (12-14).
There are some reports from Iran animal fascioliasis, particularly in cattle, based on geographical and climatic variations (3, 15, 16). Also, there are no rich and valuable surveys on the molecular and spermatogenetic ability of Fasciola spp. from Iran.
2. Objectives
The current study aimed at identifying Fasciola spp. based on spermatogenesis ability and nuclear ITS1 marker in the East of Iran, and also analyzing their phylogenic relationship with population from other parts of the world using mitochondrial ND1 gene.
3. Methods
3.1. Study Population
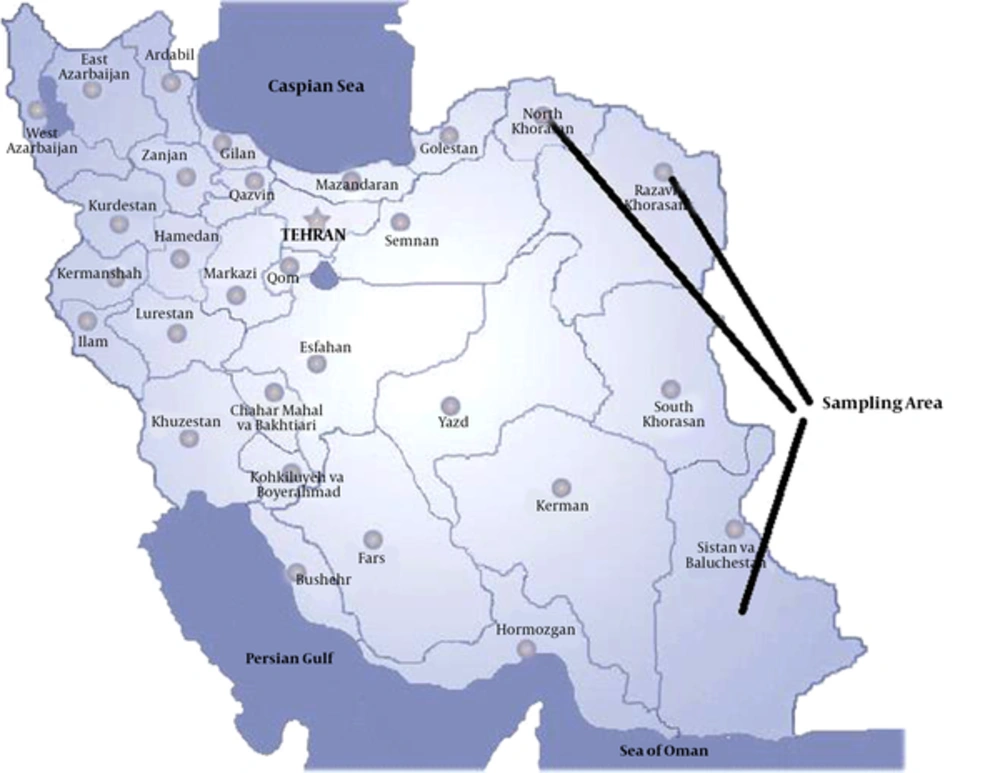
The current cross sectional study was performed on Fasciola spp. isolated from natural hosts (cattle) in the eastern provinces of Iran from January 2015 to January 2016. Sistan and Baluchistan province is noted for its unique culture and extremely dry climate, bordering with Pakistan and Afghanistan. Khorasan region includes 3 provinces with the climatic conditions similar to that of Sistan and Baluchistan province, bordering Afghanistan and Turkmenistan (Figure 1). The mentioned provinces are the poles of livestock in Iran.
3.2. Sampling of Fasciola spp. and Morphological Analysis of the Spermatogenic Status
One hundred and fifty adult flukes were collected from the bile ducts of 70 naturally infected cattle at abattoirs located in the Eastern provinces of Iran. Almost 2 flukes were randomly isolated from each host, washed with saline solution, fixed in 70% ethanol between 2 slides, and taken to the parasitology lab; then, the measurement of morphological criteria such as body length and width were carried out. The whole body of Fasciola spp. including seminal vesicle in the anterior part of the flake was dyed with hematoxylin carmine and observed under an optical microscope to examine the existence of sperm (17). Prior to staining, a small posterior part of the fluke was used for DNA extraction (Table 1).
| Fasciola spp. Fluke Population | Number of Flukes | DNA Types | ||||||
|---|---|---|---|---|---|---|---|---|
| Province | District | Number of Infected Cattle | Total | Spermic | Aspermic | ITS1 | ND1 | CO1 |
| Khorasan Razawi | Ghochan and Mashhad | 10 | 20 | 20 | - | F.g | F.g | F.g |
| North Khorasan | Bojnurd | 8 | 16 | 16 | - | F.ha | F.h | F.h |
| F.g | F.g | F.g | ||||||
| North Khorasan | Maneh | 8 | 16 | 16 | - | F.ha | F.h | F.h |
| F.g | F.g | F.g | ||||||
| North Khorasan | Shirvan | 4 | 12 | 12 | - | F.g | F.g | F.g |
| Sistan and Baluchistan | Zahedan | 16 | 48 | 48 | - | F.g | F.g | F.g |
| Sistan and Baluchistan | Zahedan (2) | 11 | 22 | 22 | - | F.g | F.g | F.g |
| Sistan and Baluchistan | Saravan | 3 | 15 | 12 | 3 | F.g | F.g | F.g |
Abbreviations: F.g, F. gigantica; F.h, F. hepatica.
aCoexistence of F. hepatica and F. gigantica in most cattle.
3.3. DNA Extraction and Amplification
Total DNA was extracted from a sample of Fasciola sp. using high pure PCR template preparation kit (Dynabio®, Takapouzist, Iran), according to the manufacturer’s instructions, and stored at -20°C until use. The ITS1 region, as a nuclear marker, was amplified with primers named ITS1-F and ITS1-R and fragments of mitochondrial target (ND1) by polymerase chain reaction (PCR) (18, 19). Total volume of the reaction was 40 µL containing 4 µL DNA template, 14 µL distilled water, 10 pmol of each primer, and 20 µL master mix (amplicon®). Reaction cycles consisted of an initial denaturation at 94°C for 90 seconds, followed by 35 cycles at 94°C for 90 seconds, 53°C (ITS1) or 55°C (ND1) for 90 seconds and 72°C for 120 seconds, with a final extension step at 72°C for 10 minutes using a gradient thermocycler. DNA fragments were visualized and analyzed by 2% agarose gel electrophoresis (20).
3.4. PCR-RFLP Method
The ITS1 marker was used to identify Fasciola spp. in PCR-RFLP (restriction fragment length polymorphism). According to the manual, DNA was digested using restriction enzyme RsaI (Cinagen®, Iran) with reaction buffer under in vitro conditions, and then, analyzed by gel electrophoresis (21).
3.5. Genetic Diversity Indices, Phylogenetic Analysis, and Sequences
Products of ND1 gene of specimens sequenced using the same primers in PCR reaction by Bioneer Company. The sequences were aligned and compared with available and existing sequences from the region in the GenBank, related to Fasciola spp. using BLAST 2.0 and ClustalW with default parameters. Phylogenic analyses based on ND1 sequence data were conducted by maximum likelihood (ML) using MEGA6 (22). Diversity indices and neutrality indices were estimated by Dnasp software package version 5.10 (23). The degree of gene flow (gene migration) among the populations was evaluated using a pairwise fixation index (Fst) (24).
4. Results
4.1. Microscopic Observation
Both spermic and aspermic Fasciola spp. were detected in the studied geographical region. One group of flukes from imported cattle in Sistan and Baluchistan province were aspermic and the remaining were spermic (Table 2). Length to width ratio in the spermic F. hepatica and spermic F. gigantica as a morphological criteria showed a significant difference (P < 0.05).
| Species | Population | Diversity Indices | Neutrality Indices | |||||
|---|---|---|---|---|---|---|---|---|
| n | Hn | S | Hd ± SD | π | Tajima’s D | Fu’s Fs Statistic | ||
| F. hepatica | East of Iran | 60 | 9 | 17 | 1 ± 0.052 | 0.01668 | -1.26770a | -3.138 |
| F. hepatica | Egypt | 19 | 14 | NC | 0.978 ± 0.027 | 0.08217 | -2.50218 | 1.298 |
| F. hepatica | China | 6 | 6 | NC | 1 ± 0.096 | 0.00586 | 0.12841 | -3.178 |
| F. gigantica | East of Iran | 90 | 4 | 22 | 0.867 ± 0.129 | 0.02689 | 0.74707 | 3.366 |
| F. gigantica | Eastern Indiaa | 91 | 32 | NC | 0.751 ± 0.050 | 0.00242 | NC | NC |
| F. gigantica | Nepala | 20 | 10 | NC | 0.753 ± 0.101 | 0.00366 | NC | NC |
| F. gigantica | Bangladeshb | 20 | 15 | NC | 0.832 ± 0.075 | 0.00362 | NC | NC |
Abbreviations: Hd, haplotype diversity; Hn, number of haplotypes; NC, not calculated; Nd, nucleotide diversity; S, number of variable sites.
aStatistical significance: not significant, P > 0.1.
bCited from Hayashi et al. (9).
4.2. Molecular Findings
The amplicons of ITS1 (approximately 680 bp) obtained from all of the spermic and aspermic flukes were cut using RsaI endonuclease digestion. RFLP patterns for F. gigantica and F. hepatica were 360 170 and 60, and 360 100 and 60, respectively. The ND1 fragments (approximately 535 bp) were amplified for all specimens.
4.3. Phylogeny and Genetic Diversity
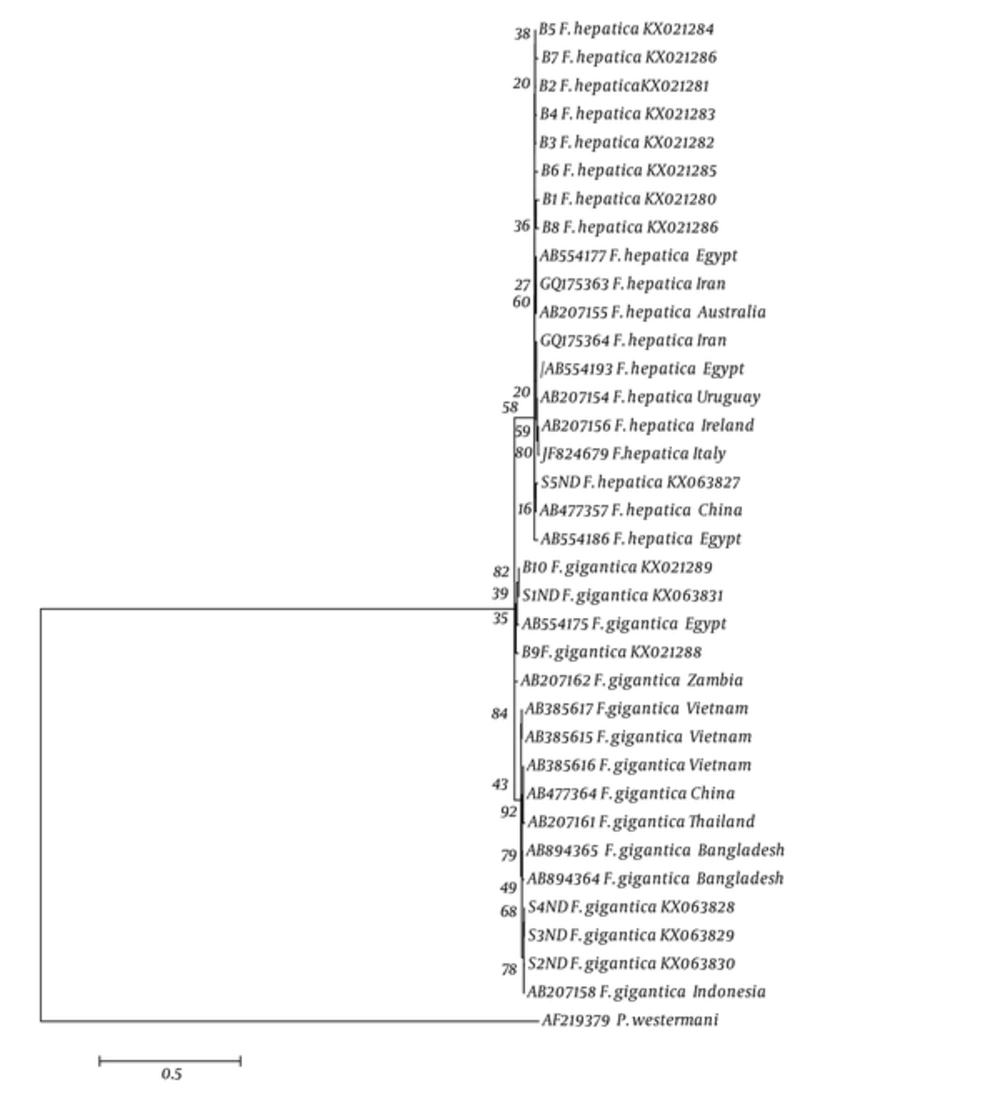
Haplotype diversity, nucleotide diversity, and variable sites of Fasciola spp. in the East of Iran, based on ND1 gene, were compared with those of other countries are shown in Table 3. The nucleotide sequences for each haplotype were deposited in GenBank under the following accession numbers: KX021280-KX021299 and KX063827- KX063836. Phylogenetic analyses based on mtDNA (ND1) sequence data were conducted by ML with lung fluke; Paragonimus westermani designated as outgroup is shown in Figure 2. Fst values between various populations of F. gigantica were calculated by Dnasp5 software package with the nucleotide data set of ND1 gene. This index was statistically significant in different F .gigantica populations and showed the genetic difference in pairwise population (Table 3). In addition, this index was statistically significant between different F. hepatica populations, except in Asia and East of Iran (Table 4 and Figure 3).
| Population | Population | ||||
|---|---|---|---|---|---|
| East of Iran | Egypt | Bangladesh | Zambia | Vietnam | |
| East of Iran | - | ||||
| Egypt | 0.98798 | - | |||
| Bangladesh | 0.99075 | 0.99599 | - | ||
| Zambia | 0.99133 | 0.98401 | 0.53823 | - | |
| Vietnam | 0.99133 | 0.99704 | 0.68867 | 0.54111 | - |
aAll values are statistically significant (P > 0.05).
aAll values are statistically insignificant (P > 0.05).
bAsia: China, Thailand, Japan.
cEurope: Italy, Poland.
5. Discussion
The differentiation of Fasciola species is crucial because of their epidemiology patterns. Fascioliasis is one the most important global concerns both for public and veterinary health. All the specimens of the current study were obtained only from the cattle that were traditionally nurtured.
Both F. hepatica and F. gigantica were prevalent in the East of Iran. The existence of both species from cattle in the North East of Iran owed to the coexistence of sheep and cattle.
Periago et al. demonstrated that the ratio of body length and width (BL/BW) is one of the useful criteria for diagnosing of Fasciola species (25), however, the current study used molecular methods to discriminate the species. A morphological report from North of Iran indicated the existence of intermediate forms of Fasciola (6), but the morphological findings of the current study did not show the existence of the intermediated form in the East of Iran, although aspermic F. gigantica was detected.
The current study aimed at finding the aspermic forms of Fasciola app. because of bordering Pakistan and Afghanistan. The study found aspermic Fasciola spp. in 2 infected cattle from Saravan in Sistan and Baluchistan province. Some reports showed the Fasciola sp. taxa in South West of Asia, in India and Bangladesh, near the studied region of Iran, but The Fg type of these flukes was detected in aspermic Fasciola sp. using ITS-RFLP, and also the phylogenetic study with ND1 gene showed that they were placed in F. gigantica complex (8, 9). These flukes were probably considered as abnormal F. gigantica with oligozoospermia, which might have occurred because of the aging of flukes. This finding was also shown in the research by Mohanta from Bangladesh (17). Studies showed that molecular phylogeny with mitochondrial DNA can be effectively used for appropriate differentiation of haplotypes (12, 18, 20).
Iran is a vast country and multiple factors may affect the haplotypes. The existence of several haplotypes in this region of Iran demonstrated the variables of ecology and climate, and the needs to provide other studies from multiple regions of Iran.
Genetic diversity in this region of Iran is high. Genetic diversity may have occurred due to free trade of cattle and transporting them through the borders of Iran with other neighboring countries. Further studies on larger populations are needed to find the reasons.
In the current study, the Fst values among 5 populations of F. gigantica ranged from 0.54111 to 0.99599, which suggested that F. gigantica populations were genetically differentiated (Table 3). These results could be related to the presence of several diverse haplotypes of the studied populations and no transfer of alleles from one population to another through the immigration of natural hosts in the region.
The Fst values showed that F. hepatica population in 3 continents was genetically different from each other, based on ND1 sequence (Table 4).
In conclusion, the current study illustrated that F. gigantica in the East of Iran has different genetic structures from the other Fasciola populations according to genetic index, but to identify the genetic diversity, further molecular studies should be performed in other regions of Iran.