1. Background
Helicobacter pylori are gram-negative spiral-shaped bacteria that colonize the gastric mucosa lining and tend to cause upper GI diseases (1). Although they can be present in 50% of the population worldwide, studies show that in developing countries, H. pylori infection is well over twice the population in developed countries (2). Some studies suggest that these bacteria can also be linked to non-gastroenterological diseases, such as ischemic heart disease, idiopathic thrombocytopenic purpura, and iron deficiency anemia (3-9). Risks for the incidence depends on the environment and poor living conditions (10, 11). The risk is higher mostly due to low socioeconomics by the theory of “contagion through society” (12). Epidemiologic reviews show most infections occur during childhood (13). Birth in developing countries is correlated with higher rates of infection, specifically crowded living conditions and poor socioeconomics (14). It seems that these bacteria are typically transferred through same age groups in families, yet then to the other members (15). Recent evidences suggest the principle role for H. pylori in dermatological problems, including Reynaud, rosacea, and urticarial rosacea, which is a chronic skin condition with frequent episodic characteristics, such as flushing, skin erythema, papulopustules, and skin telangiectasias (16, 17).
Helicobacter pylori of the gastric mucosa being considered as a pathogenic factor for rosacea has been controversial. Many reports have been published on the prevalence of H. pylori infection among patients with rosacea (11, 18-23), which ranges from studies reporting prevalence of 100% to studies indicating no significant difference between healthy people and the rosacea group (24, 25). Despite intense investigations on the spread of rosacea, the precise etiology remains unclear. There are some theories that suggest that H. pylori is a predisposing factor for occurrence; nevertheless, different results have been retrieved. Therefore, considering this controversy mentioned in the literature, in the present study, it was aimed to show the incidence of H. pylori infection in patients with Rosacea.
2. Methods
This analytical-descriptive study was conducted on patients with rosacea disease attending Tabriz University of Medical Sciences dermatology clinics from October 2011 to January 2011. Patients from age 18 to 65 years with confirmed rosacea diagnosis were included in the study. In the case of any other dermatologic problem, former H. pylori eradication treatment, allergies to omeprazole or clarithromycin, topical treatment of rosacea in the past 3 weeks, antibiotic therapy within the past 3 months, and history of hospitalization in the past 6 months, patients were excluded from the study. All included patients, were precisely examined by a dermatologist to estimate the severity of rosacea, and were categorized to 3 grades of mild, moderate, and severe, according to the classification method proposed before (Table 1) (26). All demographic information, such as age, gender, and marital status, was collected at patients’ enrollment time.
| Classification | |
|---|---|
| Mild | 1. Intermittent redness on chin, hands, and nose |
| 2.Visibly broken blood vessels appearing on skin | |
| 3. Foreign body sensation in eyes in some cases, gritty sensation and feeling irritated | |
| Moderate | 1. Persistent redness on facial skin |
| 2. Macules appearing on skin, Acne-like breakouts | |
| 3. Skin begins to thicken, Visible broken blood vessels (spider veins) | |
| 4. Reddish eyes, bloodshot appearance | |
| Severe | 1. Progressive skin redness |
| 2. Erythema swelling and nose edema | |
| 3. Blurred vision, inflamed eyes and orbit |
To assess Helicobacter pylori infection, an applicator stick of 4 to 5 mm of stool was transferred in a diluent vial, and was vortexed for 20 seconds. Then, 4 drops of the vial was dispensed in ImmunoCard STAT HpSA kit (Meridian Diagnostics, Inc., OH, USA); negative and positive results were interpreted based on the manufacturer’s recommendation. Statistical analysis was performed by the SPSS software package, version 16.0, for Windows (SPSS Inc., Chicago, USA). Quantitative data are presented as the mean ± standard deviation (SD), while qualitative data are demonstrated as frequency and percentages (%). Student t-test, Fischer’s exact and Analysis of Variance (ANOVA) were used for analysis of data. P values of less than 0.05 were considered statistically significant.
3. Results
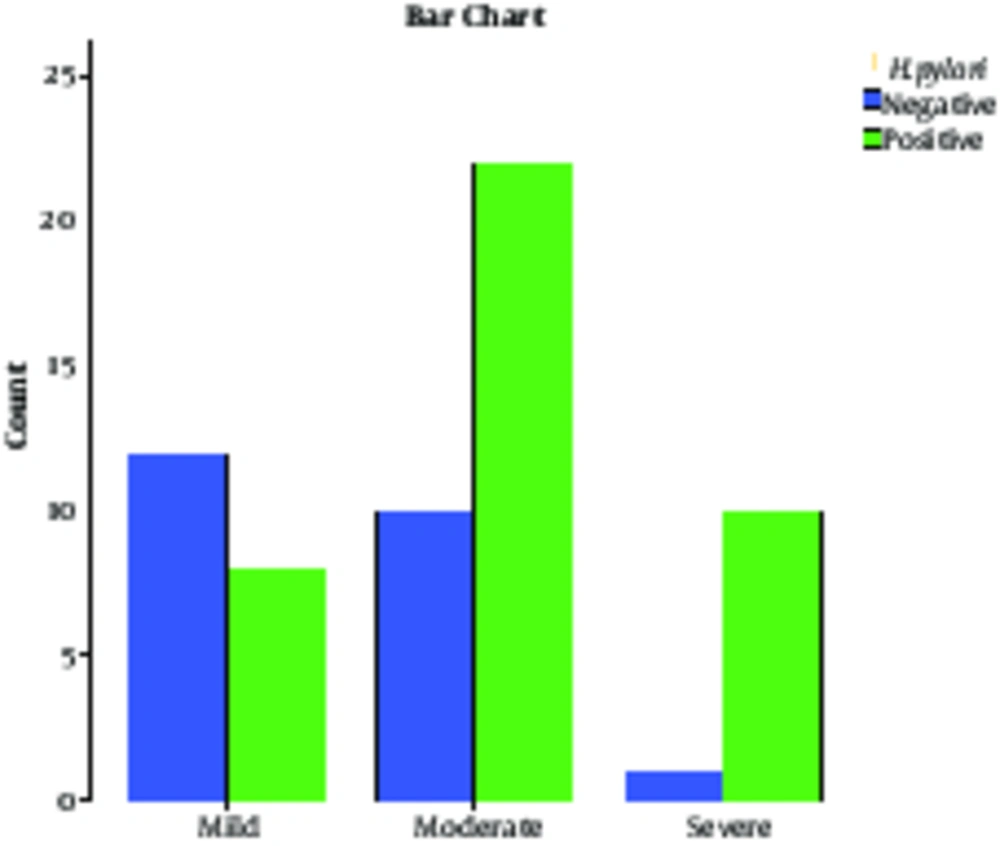
During 3 months, 63 patients were included in the study. Out of 63 patients with rosacea, 35 patients (55.55%) were male and 28 patients (44.44%) were female. The severity of rosacea among patients based on gender is shown in Table 2. The mean age of the patients was 36.46 ± 9.28 years old. Moreover, patients afflicted with severe forms were 39.9 ± 10.53 years of age, moderate forms had an average age of 35.93±10.00 and those with the mild form were aged 35.4 ± 7.04 years. There was no statistical significant difference according to age, as listed formerly (P = 0.39). In addition, 40 patients (63.4%) out of 63 were positive for H. pylori on stool antigen examination. Of all H. pylori positive patients, 24 patients (38.09%) were male and 16 patients (25.39%) were female. There was no statistically significant association between H. pylori infection and gender (P = 0.87). The mean age in patients with H. pylori positive test results was 37.02 ± 9.84 years old and in those with negative test results, mean age was 35.47 ± 8.28 years old; no statistically significant difference was found between them (P = 0.62). The severity and H. pylori antigen status are shown in Table 3; there was a statistically significant difference between patients with H. pylori negative and positive test results, considering severity (P = 0.13). This difference indicated greater H. pylori positive results in severe cases and mild cases mostly had H. pylori negative test results. Figure 1 shows the H. pylori antigen status among patients categorized based on severity.
| Female | Male | Total | |
|---|---|---|---|
| Severe | 7 (11.11) | 4 (6.34) | 11 (17.46) |
| Moderate | 15 (23.8) | 17 (26.98) | 32 (50.79) |
| Mild | 6 (9.52) | 14 (22.22) | 20 (31.74) |
| Total | 28 (44.44) | 35 (55.55) | 63 (100) |
aValues are expressed as No. (%).
| Positive H. pylori | Negative H. pylori | Total | P Value | ||
|---|---|---|---|---|---|
| Severe | 10 (15.87) | 1 (1.58) | 11 (17.46) | 0.04 | 0.013 |
| Moderate | 22 (23.8) | 10 (15.87) | 32 (50.79) | 0.43 | |
| Mild | 8 (12.69) | 12 (19.04) | 20 (31.74) | 0.01 | |
| Total | 40 (63.49) | 23 (36.5) | 63 (100) |
4. Discussion
Helicobacter pylori is one the most infectious microorganisms of human disease that is found in half of the world’s population and proved to have participated in rosacea disease development. Rosacea is a chronic dermatological disease, with an unknown pathogenesis process, which has been the subject of many studies (27). Gastric infection with H. pylori is one of the proposed pathogenic sources of rosacea establishment (28), thus many researchers have tried to examine this association by trying to investigate the correlation between H. pylori infection and rosacea. This study aimed at showing the prevalence of H. pylori in patients with rosacea. In the present study, of 63 patients with rosacea, 40 (63.4%) had positive H. pylori stool antigen. Prevalence of H. pylori varies worldwide depending on age. In a systematic review study by Eusebi et al. about epidemiology of H. pylori infection, it was concluded that in European and North American people, about one-third of adults are infected, yet in South America and Asia, the prevalence of H. pylori is often higher than 50% and there was a low prevalence of infection in the younger generations, with the authors suggesting that it may lead to a decline of H. pylori prevalence in the coming decades (29). Studies conducted in Iran regarding the prevalence of H. pylori have reported rates from 50% to 90% (30-32); thus, the results presented among patients with rosacea reveals no significant difference with the general population.
In the present study, comparing the 2 groups of H. pylori antigen negative and positive reveals a statistically greater number of severe rosacea in patients with H. pylori positive results when compared with the H. pylori-negative group. Also, the number of mild cases of rosacea in H. pylori-negative group was statistically higher than cases of severe rosacea in H. pylori-positive group. This indicates a tendency for rosacea progression among H. pylori positive rosacea patients, although there were no differences between the 2 groups of H. pylori positive and negative when considering age and gender.
In a study by Argenziano et al. the probable association between serological evidence of H. pylori infection and rosacea was investigated and it was concluded that there is a significant association between rosacea and H. pylori infection (33). However, Abram et al. evaluated several suspected risk factors for rosacea and concluded that there was no statistically significant differences between rosacea patients and those of the control group (34); the present study revealed no association between H. pylori and rosacea, while it revealed an association between H. pylori infection and severe rosacea. In an interventional study by Utas et al. about effects of H. pylori eradication on rosacea, it was shown that although H. pylori eradication treatment does not treat rosacea completely, it decreases the severity (35); this study supports the current findings, which indicates existence of an association between H. pylori infection and severe rosacea.
In a study by Crawford et al., it was proposed that there is a high probability of repetitive H. pylori positive laboratory test results among people with severe rosacea disease (36); this is similar to the current study, where severe rosacea forms were associated with H. pylori positive antigen. In a study by Alborzi et al. investigating the prevalence of H. pylori infection in children, it was concluded that H. pylori prevalence decreases with age (37). Also in developing countries, this pattern of decline has been reported; in a study by Roosendaal et al. studying the prevalence of H. pylori from the first months of birth to adolescence rates of about 20% to 10% were reported. This is in contrast with the conclusions of the current study, although the sample population was chosen among patients with rosacea.
The controversy in this field could have many etiologies. Methods of how H. pylori are diagnosed is one factor, which is different amongst various studies; diagnosis methods include H. pylori stool antigen, serum H. pylori IgG, mucosal biopsy and C-Urea breath test (38, 39). These methods, besides sensitivity and specificity, have greater differences where invasiveness and financial aspects are counted, thus each researcher may choose a method based on the circumstances, while unknowingly creating a bias. Another factor expanding this controversy is population selection; besides significant differences in H. pylori prevalence based on location and racial aspects (40, 41), most of the populations are selected in a way that selection bias occurs when including patients attending clinical centers, while most of the patients with H. pylori infection are asymptomatic.
4.1. Conclusions
In conclusion, there is no association between rosacea and H. pylori infection, although there was a significant association between H. pylori infection and severe rosacea. The current study showed no difference between groups considering age and gender. Results of the present study could be an outline for further interventional and cohort studies, which could lead to a more precise conclusion. Further studies are encouraged with a larger population considering other probable confounding covariates.