1. Background
Vulvovaginal candidiasis (VVC) is the second most frequent infection of the female genital tract, worldwide, which is indicated by irritation of vulva, vagina, or both (1). Common risk factors related to vaginal candidiasis are recent antibiotic use, diabetes mellitus, pregnancy, oral-contraceptives, and inadequate therapy (2). Different studies have shown that the extensive use of broad spectrum antibiotics and increased cases of immunocompromised patients, the prevalence of this infection is increasing all around the world (3, 4).
Approximately, 75% of women experience at least one episode of VVC during their life time and 5% experience recurrent VVC (5). Almost one-third of infected patients are diagnosed with different candida species. Although candida species in females are part of the normal vaginal flora without any clinical presentation, Candida albicans was the most frequent pathogen among other Candida species and accounts for 80% to 90% of the fungal isolates (6, 7). During the last decade, researchers find that the involved candida species are changing and infections caused by non-albicans species are increasing (8-11).
Fluconazole was the first antifungal drug for VVC therapy, and unfortunately during the recent years, different studies have shown increasing fluconazole resistance in some candida species (9, 10, 12). Candida glabrata, Candida krusi, and Candida parapsilosis show more resistance to antifungal drugs. Previous studies showed the increasing rate of Candida glabrata infection. This increase could have different reasons, such as extensive and long-term utilization of antifungal drugs (13-15). As a result, it seems that differentiation of diverse species of candida in the laboratory is very important (16).
Furthermore, VVC infection affects physical and psychological health of patients as well as economic effects and difficulties for marital relationships, and could be the cause of infertility (17).
2. Objectives
The purpose of the current study was determination of different species of candida in the Iranian female population from Damavand, although there are many studies about VVC in several regions of Iran.
3. Methods
This study was a descriptive cross-sectional study conducted in Damavand city, Iran, on patients, who had referred to the gynecology clinic of Sevom-Shaban Hospital between March, 2016 and March, 2017. The participants were women aged 20 to 60 years old with signs and symptoms, such as dyspareunia, erythema, and itching of vulva, vagina, or both, and cheesy vaginal discharge suggestive of fungal vulvovaginitis.
A standardized questionnaire was completed for each patient and the cases were asked about their age, gender, marital status, use of contraceptives, duration of symptoms, current condition signs and symptoms, pregnancy prevention methods, and antibiotic consumption history. Patients with four or more discrete attacks of VVC per year were considered as having recurrent vulvovaginal candidiasis (RVVC).
3.1. Vaginal Samples
Vaginal secretions were obtained by using a speculum and sterile swabs. The swabs were transported to the laboratory in normal saline. Two specimens were obtained under sterile conditions, one for fungal culture and the other for microscopic examination. For each of the samples, a slide was prepared for Methylene blue staining. Vaginal swabs were inoculated on Sabouraud dextrose agar with chloramphenicol (SC) and Sabouraud dextrose agar with chloramphenicol and cycloheximide (SCC) (Merck, Germany) and incubated at 30ºC. The cultures were examined after 48 hours (18, 19).
3.2. DNA Extraction
For molecular identification, DNA extraction was done with chloroform from fresh culture colonies, using the method described by Makimura et al. with some changes (20). Briefly, a loopful of fresh colony was placed in 100 μL of lysis buffer (100 mM Tris-HCl, pH = 7.5, 30 mM EDTA, 0.5% w/v SDS). Samples were incubated for 15 minutes at 100ºC and mixed with 100 μL of 2.5 M sodium acetate, kept at -20ºC for 60 minutes and centrifuged at 14000 rpm for five minutes. The supernatants were removed and DNA was precipitated with an equal volume of isopropanol and centrifuged at 12000 rpm for 15 minutes and then washed with 300 μL of 100% and 70% ethanol and centrifuged at 12000 rpm for five minutes. The sample was finally dried and suspended in 100 μL of ultrapure water. The final solution was kept at -20ºC until use as a template for PCR.
3.3. PCR-Sequencing
The ITS regions of rDNA gene of isolates were amplified by universal fungal primers, ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (21).
The PCR mixture contained 1 μL of extracted DNA, 10 pmol of each ITS1 and ITS4 primers, 0.4 mM dNTPs, 5 μL of 10X reaction buffer, and 1.25 U of Taq DNA polymerase in a final volume of 50 μL. PCR cycle parameters were as follows: One cycle at 94ºC for five minutes; 35 cycles of 30 seconds at 94ºC, 45 seconds at 60ºC, and 45 seconds at 72ºC; the final extension time was seven minutes at 72ºC.
All PCR products were subjected to sequencing for accurate identification of isolates. Overall, 20 μL of each PCR product was sequenced using the ITS1 and ITS4 primers by the BigDye Terminator cycle sequencing kit by Bioneer (Korea). For confirmation of species identity, the obtained sequences were compared with similar sequences in the open access NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
4. Results
The researchers studied 559 patients suspected of VVC at Sevom-Shaban Hospital of Damavand city, Iran. The mean age of the patients was 31.02 (± 6.6) years old. Out of 559 patients with vulvovaginitis, 46 (8.2%) patients had VVC. The age group of 30- to 39-year-olds had the highest frequency of VVC (44.7%) (Table 1). There was a significant correlation between age and occurrence of VVC (P = 0.02).
| Patient s Age Group | VVC, No. (%) | Total Participants |
|---|---|---|
| < 20 | 3 (6.4) | 9 |
| 20 - 29 | 18 (38.3) | 188 |
| 30 - 39 | 20 (44.7) | 239 |
| 40 - 49 | 5 (10.6) | 79 |
| 50 - 59 | 0 | 30 |
| > 60 | 0 | 14 |
| Total | 46 | 559 |
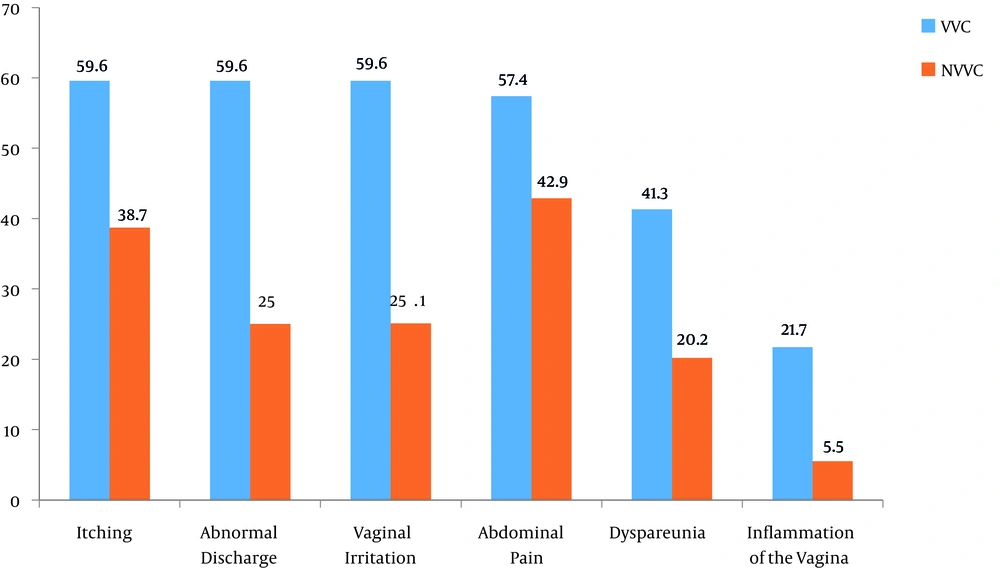
Voulvovaginal symptoms and signs in patients of VVC are demonstrated in Figure 1. The most prevalent symptoms in VVC patients were itching concomitant with abnormal discharge and vaginal irritation (59.6%). The results of the chi-square test confirmed a significant association between occurrence of the disease and dyspareunia (type of symptom) (P = 0.001). According on Fisher’s test results, a significant association was found between occurrence of the disease and two types of signs (inflammation of the vagina) (P = 0.000).
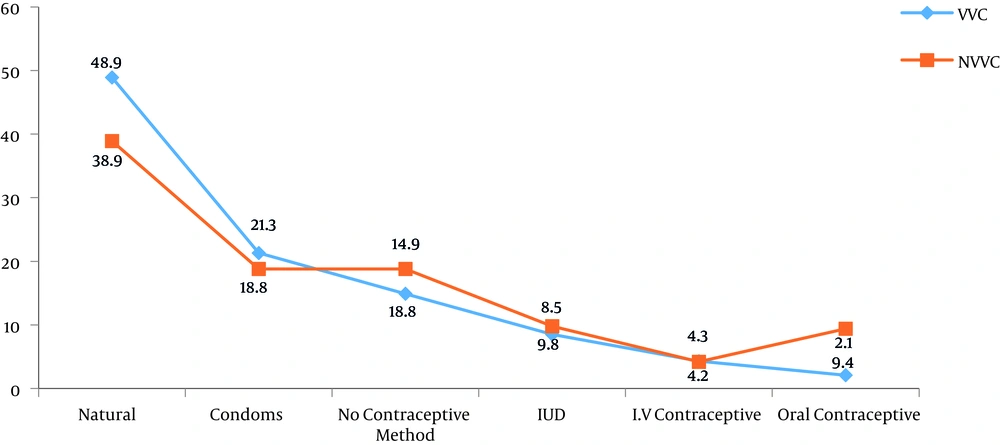
Figure 2 illustrates the distribution of studied patients based on the use of contraception methods. Most patients with VVC used the natural method of contraception (48.9%).
For the culture method, 5.5% of patients suspected of VVC had positive results among all enrolled patients. Out of 46 patients with VVC, 67.4% had positive results for Candida growth in the culture. Overall, 43 (93.5%) and 46 (100%) of these patients had positive results for KOH smear and methylene blue staining, respectively.
Fifteen patients (32.6%) had positive results in microscopic examination, which was not detected in culture.
Out of 46 patients with positive results in methylene blue staining, seven cases (15.2%) showed yeast and budding yeast and 39 (84.7%) budding yeast and pseudo hyphae. Among all patients, 31 colonies of Candida spp. were isolated from 46 patients with VVC. According to sequencing results, the most frequent was Candida albicans (67.7%), Candida glabrata (25.8%), and Candida kefyr (3.2%). Among 46 patients with VVC, one (2.1%) case showed RVVC. On the other hand, one species of Cryptococcus (Cryptococcus magnus) was isolated.
5. Discussion
Vulvovaginal candidiasis is a kind of disorder found in the presence of Candida species with signs and symptoms of vulvovaginal inflammation. This infection is the second most common cause of vaginitis symptoms after bacterial vaginitis and accounts for approximately one-third of vaginitis cases (22).
In the current study, out of 559 patients, VVC was observed in 46 (8.2%) patients. Of these patients, 4% had RVVC. The incidence of VVC varied according different studies (18, 19, 23). In the current study, the researchers found that the age group of 30- to 39-year-olds had the highest frequency of VVC, which is similar with the findings of Hedayati et al. (18) and Mahmoudi Rad et al. (23) from Iran.
In the current study, a significant correlation was found between age and occurrence of VVC (P = 0.02), in opposition to Aalei and Touhidi study (24). This may be because of higher sexual activity, higher vaginal discharge, physiological and hormonal changes, and vaginal flora changes in this age group.
In present study, the most prevalent symptoms were itching concomitant with abnormal discharge and vaginal irritation (59.6%) in VVC patients. There was a statistically significant association between occurrence of the disease and dyspareunia (type of symptom) (P = 0.001), and two types of signs (inflammation of the vagina and color of discharge) (P = 0.001).
In another study, which was conducted at Michigan university, the researchers also showed that the most common symptom in VVC is itching (25).
A prerequisite for colonization of fungi is adhesion to the epithelium, and the invasion of vaginal epithelial cells are a characteristic of this infection (26-28). In addition, damaging epithelial tissue by hyphal formation contributes to symptomatic vaginal infections (29-31). As a result, different factors could be the cause of VVC. However, vaginal pathogenicity mechanisms of Candida infection are still poorly understood.
Penetration of superficial epithelial cells due to overgrowth of the organism is associated with symptomatic disease. The mechanism of Candida species transformation, which is from asymptomatic colonization to causing symptomatic vulvovaginal disease form is complex, including host inflammatory responses and yeast virulence factors (1, 32, 33).
In the current study, VVC was mostly observed in patients, who used natural methods (48.9%) for pregnancy prevention. There was a significant correlation between the contraceptive method and disease acquisition (P = 0.001). Hedayati et al.’s study is consistent with the current result (18), however, it differs from some previous studies (11, 34). It may be assumed that the chances of VVC are increasing by this non-protective method. In addition, according to the current findings, 5.5% of patients, who were suspected of VVC had positive culture results. This percentage is in agreement with Torabi and Amini results (35), who reported a culture positivity of 4.8% in Zanjan, yet other studies (9) demonstrated a higher positivity than that observed in the current study. This variation of results may be related to different sampling criteria, and climate and geographical conditions.
In the present study, 67.4% of samples with VVC showed Candida growth in culture. The prevalence of C. albicans and non-albicans species of Candida was 67.7% and 29%, respectively. According to previous studies from Iran and different countries, the most involved species of Candida in VVC patients was C. albicans (36-38).
In the current study, the second leading species was C. glabrata (25.8%) that caused infection, and this observation was confirmed by many previous studies (39, 40).
Important virulence attributes in C. albicans include the ability to grow in both yeast and hyphal forms and the production of secreted proteinase activity (41). Candida glabrata has the ability to cause disease, independent of both of these, since it does not secrete proteinase activity and apparently cannot make true hyphae (42). However, according to genetic analysis , the C. glabrata Yapsin (YPS) genes are required for cell wall integrity, adherence to mammalian cells, survival in macrophages, and virulence.
One of the reasons, which can be the cause of the increased prevalence of non-albicans species of Candida in VVC patients is poor response of C. glabrata to azole agents, especially fluconazole (43). In the current study, prevalence of C. kefyr isolate was (3.2%), collected from VVC patients, was the same as other studies (8, 18).
In conclusion, in the vagina, yeasts were commonly found and the predominant ones are C. albicans. Many factors are imperative for final diagnosis, like correlation of vaginal examination and clinical manifestations with the isolation and identification of the yeast. Predisposing factors should be eradicated, when possible, for best therapeutic approaches.