1. Background
Tuberculosis (TB) is one of the 10 leading causes of death worldwide. In 2015, about 10.4 million people infected with TB and 1.8 million died from the disease. Over 95% of TB deaths occur in low- and middle-income countries (1).
In 2015, in Iran the total number of the cases of TB was 10,399, which 80% of them were confirmed by bacteriological methods. Due to time consuming nature of sputum culturing, sputum microscopy is helpful in the diagnosis and monitoring TB. The sensitivity of a single sputum AFB smear is 30% to 40%, but increases to 65% to 80% with multiple specimens or concentration of sputum (2). Three-sputum smear grading increases sensitivity (3). Sputum test shall be used as a first method for diagnosis, according to the Iran’s national tuberculosis guideline (4). Follow-up treatment is done with sputum culture. Sputum culture should be done monthly until culture becomes negative. If mycobacterium cultures are not practical, sputum microscopy in the 2, 3, and 5 months of treatment should be done (5). The laboratories have important roles in monitoring and controlling TB in the world (6). The current study aimed at identifying directly observed treatment short course (DOTS) performance and the rate of AFB-positive and AFB-negative after a 2-month treatment with isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (ETM) in the Sanabad health center of Mashhad, Iran from March 2005 to March 2008.
2. Methods
The current analytic, cross sectional study used recorded programmed data.
The study was carried out in Sanabad Health Center of Mashhad; the second most populous city in the Islamic Republic of Iran and the capital of Khorasan Razavi province. It is located in the Northeast of the country, near the borders of Turkmenistan and Afghanistan. Its population was 2 749 374 in 2011. It has an area of 328 km2 and population density of 8 400/km2 (7).
Department of provincial health center is a secondary referral medical center that examines an average of 10 smears per day by the acid-fast bacilli (AFB) staining, and DOTS to the patients.
Patients older than 18 years with new pulmonary TB positive-sputum who underwent treatment with 4 drugs (INH, RMP, PZA, and ETM) from Marche 2005 to March 2008 were included in the current study. Symptom criteria were defined clearly. The most common symptoms of pulmonary TB were cough for 2 weeks, sputum, bloody sputum, weight loss 5% to 10% of body weight, fever, shortness of breath, chest pain ,back pain, night sweating, fatigue, and asthenia.
Exclusion criteria were patients with incomplete and discontinued treatment, diabetes mellitus, HIV infection, AIDS, old age (> 65 years), age less than 18 years, cavity, thrombocytosis, and death. The traditional method (light microscopy examination of the specimens stained with Ziehl-Neelsen basic fuchin) was used. Ziehl-Neelsen smears were prepared with 0.32% carbolfuchsin, heated gently for 5 minutes until steam rose, washed with tap water, flooded with 3% hydrochloric acid-alcohol for 1 minute, and washed and flooded with 1% methylene blue for 1 minute. Slides were examined by a single technologist using a 100X oil immersion objective, screening at least 100 fields, with positive and negative control slides examined twice per week (8). Smear microscopy was performed by a person trained in Sanabad health center.
A total of 700 cases of tuberculosis referred to Sanabad health center in 36 months from Marche 2005 to March 2008 were studied. The random sampling method was employed and the data distribution was normal. The sample size was determined using the following formula based on 95% confidence interval (CI), 5% relative error (RR), and P value of 0.8
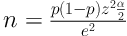
According to the formula, 246 patients were enough. Since the study period was 36 months, 360 patients were included and 340 patients excluded. The variables were gender, age, nationality, and smear microscopy results. Variables were collected on the basis of information contained in patient records. Data were collected by a same person.
Demographic data including age, gender, nationality, and sputum microscopy results of the patients were collected in datasheets anonymously. Statistical analysis and comparison of the rate and mean were conducted with SPSS software version 18 using the chi-square and t tests. P values less than 0.05 were considered statistically significant. The results were shown in charts and tables. Ethics approval was obtained from the ethics advisory group of the International Union Against Tuberculosis and Lung Disease, Paris, France, and the ethical committee of Iranian Ministry of Health in Mashhad, Iran (code: IR. Mums.REC.1395.628).
3. Results
Table 1 shows the distribution of patients’ gender, 40.3% were male and %59.7 female; the gender ratio was 67.4.
| Gender | No. (%) |
|---|---|
| Male | 145 (40.3) |
| Female | 215 (59.7) |
| Sum | 360 (100.0) |
Table 2 shows that 26.1% of the patients were younger than 25 years, 16.9% ranged 25 to 35 years, 11.7% 36 to 45 years, and finally 45.3% of them were older than 46 years.
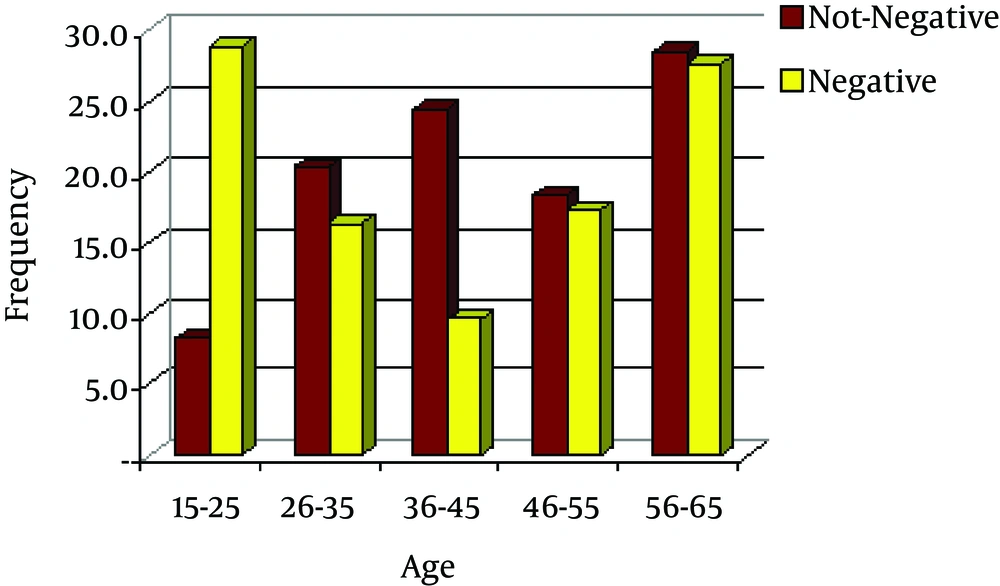
Age distribution of 360 patients was in 5 age groups; the highest prevalence was in the age group 56 to 65 years followed by 15 to 25 years.
| Age, year | No. (%) | Accumulation Rate |
|---|---|---|
| 15 - 25 | 94 (26.1) | 26.1 |
| 26 - 35 | 61 (16.9) | 43.1 |
| 36 - 45 | 42 (11.7) | 54.7 |
| 46 - 55 | 63 (17.5) | 72.2 |
| 56 - 65 | 100 (27.8) | 100.0 |
| Total | 360 (100.0) | - |
Table 3 shows the rate of conversion of sputum smears; 49 patients were (13.6%) AFB-positive and 311 AFB-negative (86.4%) (P < 0.001).
| Sputum Smear | No. (%) |
|---|---|
| AFB-positive | 49 (13.6) |
| AFB-negative | 311 (86.4) |
| Total | 360 (100) |
Table 4 shows that the mean ages of the patients with AFB-positive and AFB-negative were 44.8 and 40.8, respectively, which statistically had no difference (P value = 0.123).
There was no gender difference in patients with AFB-negative (P value = 0.1815).
| Sputum Gender | AFB-Positive | AFB-Negative |
|---|---|---|
| Male | 24 (49.0) | 121 (38.9) |
| Female | 25 (51.0) | 190 (61.1) |
| Total | 49 (100) | 311 (100) |
The differences between gender and AFB-positive/AFB-negative sputum were statistically insignificant (P value = 0.1815).
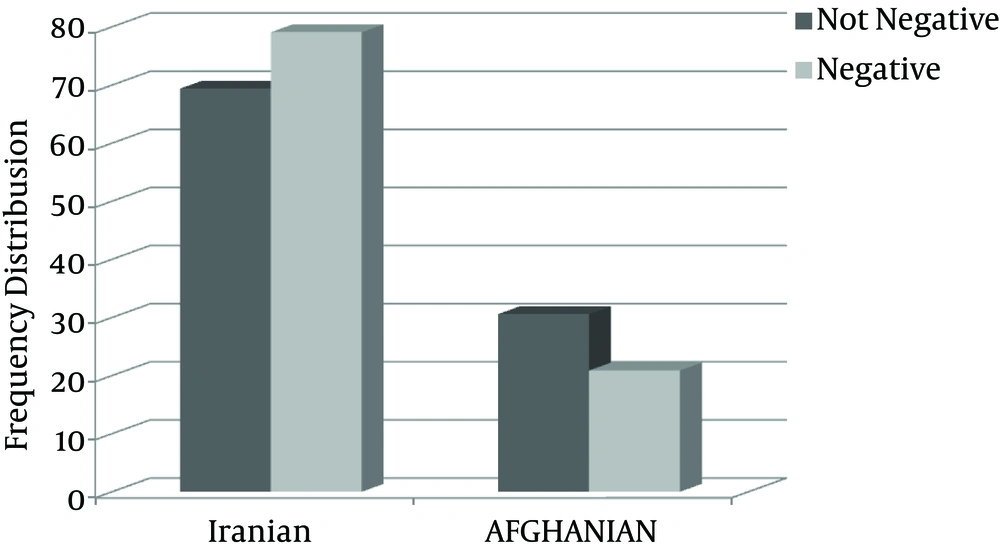
It should be mentioned that there was no statistically significant relationship between the rate of sputum smears conversion and the nationality of the patients (P value > 0.05).
Figure 1 shows the frequency of sputum smear AFB-positive/AFB-negative with age.
Figure 2 the shows frequency of relationship between sputum smears and the nationality.
4. Discussion
TB has occurred for millennia and still is a major global health problem. It causes illness in millions of people each year and in 2015 was one of the leading 10 causes of death worldwide, ranking above HIV/AIDS as one of the leading causes of death from an infectious disease (1).
Although TB is largely a curable disease, it remains a major cause of morbidity and mortality worldwide (9). Sputum smear microscopy was developed more than 100 years ago. Sputum samples are examined under a microscope to observe bacteria. In the current case, definitions recommended by the world health organization (WHO), one positive result is required to diagnose smear-positive pulmonary TB (1). There were developments in diagnostic methods in recent years with the introduction of polymerase chain reaction (PCR), viability, and stain microscopy with fluoresce in diacetate, but culture, microscopy sputum, and weight gain were used in low-resource setting to monitor response to the treatment of new smear-positive TB cases.
Serial chest radiographs are not recommended. Nucleic acid amplification technology is not suitable to follow-up (5). Nucleic acid amplification cannot be substituted by clinical judgment, acid-fast smear, and culture to diagnose TB (3). In 2015, for example, 57% of the TB pulmonary cases reported to WHO were bacteriologically confirmed (1).Smear microscopy is simple, cheap, rapid, and easy to perform method, and does not require complex laboratory equipment. Smear microscopy reflects the extent of disease with higher smear grades, which is associated with a more extensive lung involvement and the presence of cavity lesions. Quantitative assessment of sputum specimens is done microscopically. Sputum quality should be considered and with enhanced routine supervision of laboratory and training, TB can be stopped (10).
Determination of the incidence of smear-positive tuberculosis can play an important role in promoting the TB control program (11).
Without treatment, the death rate due to TB is high. Studies on the natural history of TB disease in the absence of treatment with anti-TB drugs (conducted before the availability of drug treatments) showed that about 70% of people with sputum smear-positive pulmonary TB died within 10 years, as did about 20% of people with culture-positive (but smear-negative) pulmonary TB. At 2 months follow-up, the sputum examination result is necessary to decide on whether to continue the intensive phase of treatment for more 1 month in case of positive results, or move to the second phase of treatment in case of negative results (12). The main diagnosis method of TB laboratories in the developing countries without the facilities and equipment to culture the samples was the preparation and direct microscopic observation; although the sensitivity of staining AFB is lower than that of the culture and it greatly depends on the skill of the tester and using appropriate methods, it is the quickest and most immediate method of TB diagnosis, especially in patients with very high microbial load and the high risk of transmission to others, or to detect patients in need of treatment (13).
The current study monitored 360 patients after 2 months of treatment with INH, RIF, PZA, and ETM. The majority of the study patients were female. In 2016 global data, the gender ratio of male to female was 1.6:1 (1). Sex hormones could be a significant factor for this difference, considering that testosterone impairs macrophage activation and pro-inflammatory cytokines production, while estrogens are pro-inflammatory mediator inducer (14). Boushab in Hodh EI Garbi studied 320 cases and found a statistically significant relationship between gender, age group, and resistance to TB after 6 months of anti-TB treatment (15). Treatment results were better in females with higher education than in similarly educated males (16). In some of the studies, the sputum smears converted to negative, slightly later, in males. In the current study, 40.3% of the patients were male and 59.7% female and among 49 AFB-positive after 2 months, 24 patients were male; 121 of the AFB-negative cases (39%) were male, but there was no statistically significant difference between gender and sputum conversion (P value = 0.1815). Unsematham in a 3-year epidemiological retrospective cohort in 2012 in Thailand indicated that the proportion of patients with TB who remained smear-positive after 2 months of treatment could be greater than 20%. The lack of smear conversion in the second month of treatment was one of the predictors of treatment failure and relapse. Three hundred fifty-six patients were included in the study. Male gender, high initial sputum acid-fast bacilli grade, and cavitary diseases were the factors associated with the lack of the 2-month positive smear and increasing the risk of treatment failure (17). In the current study, females were more than males; perhaps females paid more attention to their health.
In the current study, among 360 examined patients, 26.1% were less than 25 years, 16.9% 25 to 35 years, 11.7% 36 to 45 years, and finally, 45.3% of the patients were above 46 years. The mean age of the patients with AFB-positive after 2 months was 44.8 years, while the mean age of the patients with AFB-negative after 2 months was 40.8 years, and 45.3% of the subjects were above 46 years; no statistically significant difference was observed (P value = 0.123).In the study by Nassiri on 6426 patients in Iran, the mean age of the patients was 45 years (18). In the study by Soudbakhsh in Emam Khomeini hospital of Tehran, definitive relationship between the change sputum smear and age was not reached (19).
Wang, in a study of factors influencing time to smear conversion in patients with smear-positive pulmonary tuberculosis revealed that 11.1% of patients with TB remained smear-positive after 2 months of treatment. Patients with cavity, higher smear grading, and the ones that did not use INH, RIF, ETM, and PZA continuously in the initial treatment phase had a longer time for sputum smear conversion (20).
Chaudhry conducted a prospective cohort on 100 newly diagnosed patients with open pulmonary TB in Dammam, Kingdom of Saudi Arabia from July to December 2010; the overall sputum conversion in the first month was 56% and 76% in the second month, and 94% in the third month. In the study by Rathman et al. in Gambia on 340 adults with smear-positive tuberculosis, wasting was the most common clinical sign of sputum-positive tuberculosis (21).
Pajankar found that the sputum conversion rate among the patients with tuberculosis treated with DOT for 1 and 2 months in Oman was 34.8% and 78.6%, respectively in 2007. Platelet count could assist in predicting early sputum conversion (22).
The next factor was the nationality of the patients, in which Afghan nationality was attributed to the rate of sputum smears conversion from positive to negative. In the current research, among 49 positive-sputum smears, 34 (69.4%) were Iranian and 15 (30.6%) Afghan tested by Chi-Square test and no significant difference was detected among them (P value = 0.129).
Among 360 studied patients, 49 cases (13.6) had sputum smears positive after 2 months of treatment and in the remaining 311 (86.4%) the sputum smears converted to negative after 2 months. It should be noted that according to the test, treatment success rate was at least 85% of the reported rate by WHO in 2016. The importance of conversion smear rate in 2 months after treatment shows the performance of DOTS and prevention of infection in the community. The lack of attention paid to the quantity and quality of sputum smear were the weaknesses of the current study. Positive sputum smears are much more likely to indicate M. tuberculosis than M. avium coinfection, even in the areas where both diseases are common (3).
The performance of DOTS system was examined according to the results of the presented study on the rate of sputum smears conversion to negative, and its acceptability relative to the global criteria and standards; it can be suggested that precise monitoring of the patients with TB such as monitoring the proper consumption of the drugs and disease complications and sputum smears follow-up should be performed similar to the previous trends. Also, according to the findings of the current study, the common drug administration had proper effect and its use is recommended in the patients with TB. In order to control TB in Iran, attention should be paid to the quantity and quality of sputum smear. Sputum pretreatment procedures to optimize smear microscopy should be done in Iran (23). Prospective studies in Iran on a larger number of patients, considering the clinical diseases, quantity and quality of sputum, culture sputum, PCR, and viability stain microscopy with fluorescein diacetate are suggested. Management of comorbid conditions including nutritional status could improve anti-tubercular treatment responses (9).
Iran accepts public-private mix directly observed treatment short course chemo-therapy (PPM-DOTS) strategy for TB control program. However, use of sputum smear microscopy is inadequate for diagnosis and treatment of TB (24). TB control studies should be monitored and evaluated using a TB indicator. Support for smoking cessation should be considered to improve outcomes in TB control programs (25). Arginine is useful as an adjunctive therapy in patients with active TB, in which the effects are more likely mediated by the increased production of nitric oxide and improved constitutional symptoms and weight gain (26).
Children who did not demonstrate sputum conversion after intensive phase of anti-tubercular therapy had lower baseline 25-hydroxy vitamin D levels as compared to the ones who did (27).