1. Background
Vulvovaginal candidiasis (VVC) is a common fungal infection among women worldwide. The infection is caused from the lower genital and is reported in 35% - 80% of cases without any symptoms (1). Symptomatic VVC has been found in up to 70% of the sexually active women (2). The main risk factors are antibiotic usage, hormone replacement therapy, diabetes mellitus, oral-contraceptives, pregnancy, and insufficient therapy (3). Detection of risk factors is a significant way to prevent VVC. Symptoms mostly include itching, soreness, burning, and vaginal discharges (4). Candida albicans is the most etiologic agent of VVC followed by non-albicans Candida species including C. kefyr, C. tropicalis, and C. glabrata (5). Various topical and oral antifungal agents such as ketoconazole, clotrimazole, fluconazole, and miconazole are used for treatment of Candida vaginitis (6). However, antifungal resistance among different Candida species is considered as a main factor for the recurrence of infection especially in immunosuppressed patients (7). The aim of the present study is to identify Candida species obtained from patients with vulvovaginitis by molecular techniques and evaluation of 3 antifungals for treatment of patients.
2. Methods
2.1. Specimens
In this cross sectional descriptive study (November 2015 to April 2016), clinical specimens were obtained from 108 suspected patients that were referred to the center for specialized gynecology and transferred to the medical mycology laboratory of Isfahan University of Medical Sciences, Isfahan, Iran. The inclusion criteria were non-specific signs such as vaginal discharge, itching, burning, inflammatory, as well as soreness. The exclusion criteria included antifungal consumption, menstruation, and bacterial vaginosis. All clinical variables were obtained by a comprehensive questionnaire that was filled out by each patient.
2.2. Antifungal Therapy
Suspected patients were divided into 3 groups and each group took only 1 antifungal agent including clotrimazole, miconazole, and nystatin, respectively.
2.3. Clinical Isolates
Two vaginal wet swabs were taken from each patient for direct microscopic examination and culture. All samples were sub-cultured on sabouraud glucose agar (Difco, Detroit, MI, USA) with chloramphenicol and CHROMagar Candida (Paris, France).
2.4. Molecular Identification
2.4.1. DNA Extraction
Genomic DNA was extracted using the boiling method (8). Briefly, a bit of fresh colonies were suspended in 100 μL of distilled water and boiled for 15 minutes, then centrifuged for 4 minutes at 7000 rpm, and then the supernatant was used for polymerase chain reaction (PCR).
2.4.2. Fragment Size Polymorphism (FSP) Method
ITS1 and ITS2 regions were amplified in the separate PCR reaction at the same time. PCR mixture contained: 5 μL of 10 × reaction buffer, 1.5 mM MgCl2, 0.4 mM dNTPs, 2.5 U of Taq polymerase, 30 pmol of both ITS1 (5’-TCC GTA GGT GAA CCT GCG G-3’) and ITS2 (5'- GCTGCGTTCTTCATCGATGC-3’) primers for one set of PCR reaction (9, 10) and ITS3 (5’-GCATCGATGAAGAACGCAGC-3’) and ITS4 (5’-TCC TCC GCT TAT TGA TAT GC-3’) for another (11), and 2 μL of extracted DNA in a final volume of 50 μL. The PCR cycling was composed of: initial denaturation phase at 95°C for 5 minutes, followed by 34 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 45 seconds, and extension at 72°C for 1 minutes, with a final extension phase at 72°C for 7 minutes.
2.4.3. Electrophoresis
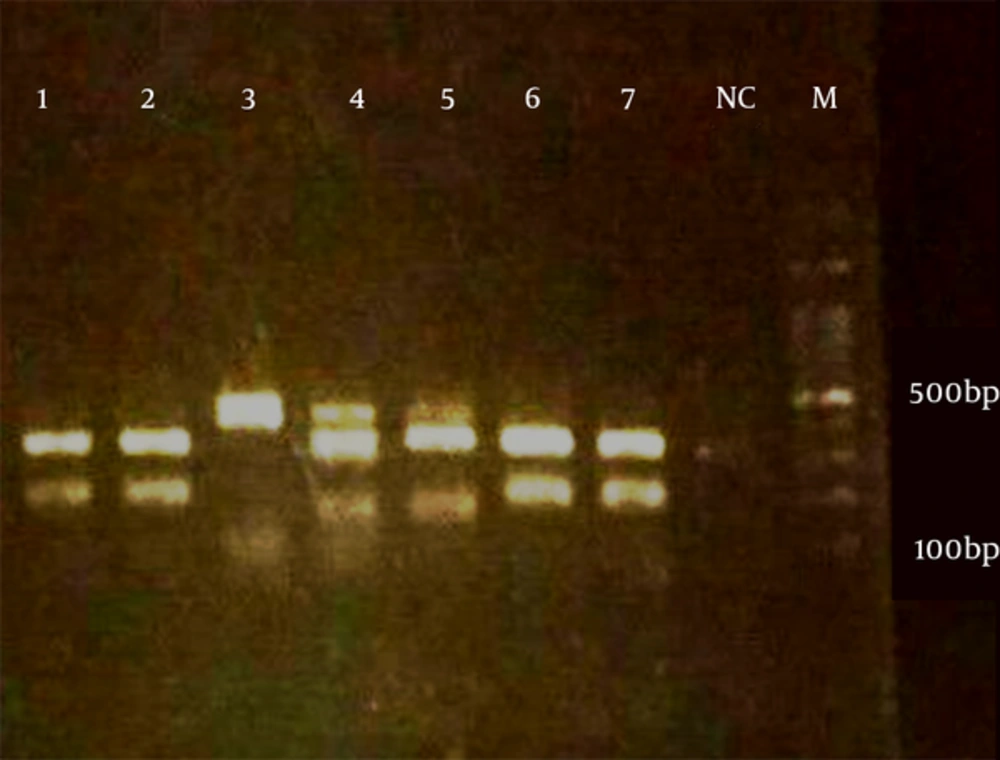
Five microliters of ITS1 and ITS2 products were blended and run onto 2% agarose gel as well as electrophoresed in TBE buffer (90 mM boric acid, 90 mM Tris, 2 mM 127 EDTA) at 10 V/cm for 60 minutes.
2.5. Statistical Analysis
Antifungal effects of clotrimazole, miconazole, and nystatin were evaluated using the SPSS software version 14.0. Comparison of antifungal agents’ effects was adjusted using the Chi-squared test and Fisher’s exact test. A P value of < 0.05 was considered significant.
3. Results
Clinical manifestations among suspected cases were pruritus (84%), burning (74%), vaginal discharge (71%), pain during or after sex (30%), and inflammatory (8%). A total of 59 out of the 108 cases (54.6%) had both a positive culture and direct microscopic examination and 49 patients were kept out from the study due to the exclusion criteria. The duration of disease was between 3 to 365 days. All patients were married, however, none of the patients were pregnant. Use of antibiotics (35.6%) and diabetes mellitus (6.8%) were the most predisposing factors among patients. Candida albicans was the most prevalent Candida species isolated from patients (74.5%) followed by Candida glabrata (17%) (Table 1, Figure 1). Molecular patterns of clinical isolates on agarose gel were in accordance with CHROMagar Candida finding except for sample number 15. Most patients were in the age group of 26 to 30 years (22%) (Table 2).
| No. | Age | Symptoms | Duration of Infection (Day) | Use of Antibiotic | Use of Antifungal | Use of Oral Contraceptive Drug | Risk Factors | Candida Species |
|---|---|---|---|---|---|---|---|---|
| 1 | 34 | Itching, Burning, Pain, Discharge | 30 | + | - | + | - | C. kefyr |
| 2 | 48 | Burning, Discharge | 14 | - | - | - | Diabetes mellitus | C. albicans |
| 3 | 41 | Itching, Burning | 60 | - | - | - | - | C. albicans |
| 4 | 33 | Itching, Burning, Pain, Discharge | 120 | - | - | - | - | C. albicans |
| 5 | 23 | Itching, Burning, Pain, Discharge | 90 | Cefixime | - | + | - | C. albicans |
| 6 | 29 | Itching, Discharge | 14 | - | - | + | - | C. kefyr |
| 7 | 20 | Itching, Burning, Pain, | 6 | - | - | - | - | C. albicans |
| 8 | 35 | Itching, Burning | 14 | - | - | - | - | C. albicans |
| 9 | 35 | Itching, Burning, Discharge | 30 | - | - | - | - | C. glabrata |
| 10 | 18 | Discharge | 14 | Azithromycin | - | - | - | C. krusei |
| 11 | 25 | Burning | 30 | - | - | + | - | C. krusei |
| 12 | 36 | Itching, Pain, Discharge, Inflation | 60 | - | - | - | - | C. albicans |
| 13 | 36 | Itching, Discharge | 150 | - | Clotrimazole | - | - | C. albicans |
| 14 | 30 | Itching, Burning, Pain, Discharge | 365 | - | - | - | - | C. albicans |
| 15 | 25 | Burning, Discharge | 7 | Azithromycin | - | - | - | C. albicans |
| 16 | 17 | Itching, Burning, Pain, Discharge, Inflation | 4 | - | - | + | - | C. albicans |
| 17 | 27 | Itching, Burning, Discharge | 210 | Metronidazole | - | + | - | C. albicans |
| 18 | 36 | Itching, Burning, Pain, Discharge, Inflation | 365 | Azithromycin+ Metronidazole | Fluconazole | - | - | C. albicans |
| 19 | 25 | Discharge | 90 | - | - | + | - | C. albicans |
| 20 | 31 | Itching, Burning, Discharge, Inflation- | 4 | Cefixime | - | - | - | C. albicans |
| 21 | 43 | Itching, Burning, Inflation | 4 | Penicillin | - | - | - | C. albicans |
| 22 | 28 | Discharge | 180 | Azithromycin | Clotrimazole | + | - | C. albicans |
| 23 | 26 | Itching, Burning, Discharge | 14 | - | - | + | - | C. kefyr |
| 24 | 46 | Itching, Burning, Pain, Discharge | 60 | Amoxicillin | - | - | - | C. glabrata |
| 25 | 20 | Burning, Discharge | 21 | - | - | + | - | C. glabrata |
| 26 | 25 | Burning, Discharge | 60 | - | - | + | - | C. glabrata |
| 27 | 27 | Itching, Burning, Pain, Discharge | 30 | - | - | - | Hypothyroidism | C. glabrata |
| 28 | 32 | Itching | 4 | Penicillin | - | + | - | C. glabrata |
| 29 | 42 | Itching, Burning, Discharge | 4 | - | - | - | Multiple sclerosis + Hypertension | C. albicans |
| 30 | 45 | Itching, Burning, Pain, Discharge | 365 | Azithromycin | - | - | - | C. albicans |
| 31 | 21 | Itching, Burning, Discharge | 5 | - | - | + | - | C. albicans |
| 32 | 39 | Itching, Burning | 21 | - | - | - | - | C. albicans |
| 33 | 40 | Itching, Burning, Pain, Discharge | 7 | - | - | - | Asthma | C. glabrata |
| 34 | 28 | Itching, Discharge | 240 | - | Clotrimazole | + | - | C. albicans |
| 35 | 37 | Itching, Burning, Pain, Discharge | 365 | - | - | - | - | C. albicans |
| 36 | 50 | Itching, Burning, Discharge | 30 | - | - | - | Diabetes Mellitus + Hypothyroidism | C. glabrata |
| 37 | 25 | Itching, Burning, Pain, Discharge | 120 | - | - | + | - | C. albicans |
| 38 | 30 | Itching, Burning, Pain, | 3 | + | - | - | - | C. albicans |
| 39 | 36 | Itching, Burning, Pain, | 7 | - | - | - | Hypothyroidism | C. albicans |
| 40 | 34 | Itching, Burning, Discharge | 30 | Cefixime+ Metronidazole | - | + | - | C. glabrata |
| 41 | 47 | Itching, Burning | 7 | - | - | - | - | C. glabrata |
| 42 | 31 | Itching, Burning, Discharge | 21 | - | - | + | - | C. albicans |
| 43 | 21 | Burning, Discharge | 30 | Cefixime | - | - | - | C. albicans |
| 44 | 29 | Itching | 4 | - | - | + | - | C. albicans |
| 45 | 29 | Itching, Burning | 30 | + | - | + | - | C. albicans |
| 46 | 38 | Itching | 365 | + | - | - | - | C. albicans |
| 47 | 29 | Itching, Burning, Discharge | 365 | - | - | + | Diabetes Mellitus | C. albicans |
| 48 | 25 | Itching, Burning, Discharge | 3 | + | - | + | - | C. albicans |
| 49 | 27 | Discharge | 7 | + | - | - | - | C. albicans |
| 50 | 37 | Itching, Discharge | 150 | Cefixime | - | - | Diabetes Mellitus | C. albicans |
| 51 | 20 | Itching, Burning, Discharge | 30 | Azithromycin | - | + | - | C. albicans |
| 52 | 46 | Itching, Burning | 7 | - | - | - | - | C. albicans |
| 53 | 40 | Itching, Discharge, Inflation | 365 | - | - | - | - | C. albicans |
| 54 | 34 | Itching, Burning, Discharge | 7 | - | - | - | - | C. albicans |
| 55 | 34 | Itching, Discharge, Inflation | 150 | - | - | - | Rheumatoid arthritis | C. albicans |
| 56 | 31 | Itching, Discharge, Inflation | 4 | - | - | + | - | C. albicans |
| 57 | 48 | Itching, Discharge | 21 | - | - | - | - | C. albicans |
| 58 | 30 | Itching, Discharge, Inflation | 90 | - | Clotrimazole | - | - | C. albicans |
| 59 | 35 | Itching, Pain, Discharge | 60 | - | Fluconazole | - | - | C. albicans |
| Age Group | No. | % |
|---|---|---|
| 15 - 20 | 5 | 8.5 |
| 21 - 25 | 9 | 15.2 |
| 26 - 30 | 13 | 22 |
| 31 - 35 | 12 | 20.3 |
| 36 - 40 | 10 | 17 |
| 41 - 45 | 4 | 6.8 |
| 46 - 50 | 6 | 10.2 |
Comparison of drug effectiveness and successful remediation of patient was adjusted using Chi-squared test and Fisher’s exact test, however, the P value was not statistically significant (P value = 0.056).
4. Discussion
Candida albicans can colonize on the mucous membrane of genitourinary tracts of healthy humans. Candida virulence factors are: adherence, enzyme production, proteinase secretion, dimorphisms with antigenic variations, and cell surface composition (12, 13). Changing from yeast to hypha is under the control of a complex set of environmental signals and has long been believed to be the main virulence factor of this pathogen (14). Compared to other species such as Candida krusei, Cancida glabrata, and Candida tropicalis, Candida albicans has the most durable binding ability (15). Phosphorylase and aspartyl protease enzymes are main agents needed for tissue invasion in the pathogenesis of the infection (3, 16). The effects of estrogen on the vaginal mucosa are main factors for infection progression. Throughout the menstrual cycle, estrogen causes the thickened and cornified keratinized vaginal epithelium (17). This is further confirmed by the fact that low estrogen producers (prepubescent girls and postmenopausal women) infrequently develop vaginitis. Vulvovaginal candidiasis show different frequency region to region and some studies have disclosed that the incidence of vulvovaginal candidiasis differs in various areas. It can be related to geographical conditions, social and cultural factors, hygiene customs, and diagnostic techniques (18, 19). The prevalence of infection has been reported as 12.1% in Athens, 17.4% in Turkey, 12.2% in Brazil, 20.4% in India, 18.7% in Israel, and 6.5% in China (3, 18-22). Candida albicans can be found in the cultures of vaginal discharge of 25% of pregnant women (16). Pregnant women have a two-fold increase in the frequency of vulvovaginal colonization by Candida spp. compared to non-pregnant individuals. This association is influenced by elevated levels of circulating estrogens as well as accumulation of some substrates like glycogen in the vagina during pregnancy (23, 24). This increased rate depends on the stage of pregnancy (the first, second, or third trimester of pregnancy), the level of glycogen, and the amount of bacterial microbiome, such as lactobacilli in the vaginal lumen that produce lactic acid and H2O2 and provides significant protection against fungal infections. Interestingly, none of the patients were pregnant in the present investigation. Masri et al. (25) reported 17.2% VVC in pregnant women. They performed traditional tests such as Gram-staining, microscopic examination, and culture for species identification and reported C. albicans as the most prevalent species (83.5%), followed by C. glabrata (16%), whereas we used a precise molecular technique (PCR-FSP) for identification of Candida species in the present investigation. To our knowledge, this is the first time that PCR-FSP is used to identify causative agents of vulvuvaginal candidiasis. Mucci et al. (26) showed that the occurrence of VVC was 25% among pregnant women and Candida albicans with a prevalence of 80.7% was the predominant Candida species. Nnadi and Singh (27) reported on 288 pregnant women, 175 were positive for VVC giving a prevalence rate of 60.8%. They also revealed that pregnant women with an age range of 26 - 30 years had the highest prevalence of infection (37.1%). Mohammadi et al. (28) identified Candida species isolated from VVC by the PCR-RFLP method in Kashan and reported Candida albicans (87.2%) and Candida glabrata (12%) as the most frequent species, in agreement of the present study. Nearly 5% - 8% (about 150 million worldwide) suffer from recurrent VVC (RVVC) (29-31). Medication of antibiotics is another major predisposing factor for expanding fungal vaginitis, supposedly through disturbance of the natural bacterial population existing at the mucosal interface (32, 33). Reduced levels of acid-producing lactobacilli causes raised vaginal pH levels and increased fungal colonization of potential pathogens (34). In accordance with this hypothesis, 35.6% of patients had used antibacterial agents in the present study. The major complaint of Candidal vulvovaginitis was itching in this survey and the usual clinical sign was vaginal discharge. In the same way, Ebrahimy et al. (16) and Grigoriou et al. (3) reported that itching was the most frequent complaint among patients with the Candida infection with the rate of 98% and 85%, respectively. Invasion to the epithelial cells in the lower genitourinary tract by Candida species can cause inflammation and itching due to the enzyme or toxin involved in the pathogenesis of Candida species. Antifungal susceptibility testing (AFST) of Candida species may provide good treatment outcome, assessment of antifungal efficiency, and monitoring of incidence of drug resistance. Unfortunately, we did not perform AFST on clinical isolates in the present study, however, suspected patients were treated empirically with clotrimazole, miconazole, and nystatin. Nystatin is used to treat Candida infections in the mouth or stomach mucosa. It is not a choice for treatment of volvuvaginal candidiasis, however, it was as effective as miconazole and clotrimazole in the present survey. Achkar and Fries (29) suggested that volvuvaginal candidiasis is often recognized without complementary tests and treated with unusual drugs, therefore the incidence of infection is unknown. Another limitation of the present study was to quit taking medication prior to the completion of treatment period, so we were not able to evaluate the drug effectiveness of antifungal agents on patients, precisely. It is highly recommended that incomplete treatment of infection be considered as exclusion criteria in same studies in the future. In the present investigation, the age range of 26 - 30 years had the highest occurrence of volvuvaginal candidiasis, which is in agreement with the findings of Hedayati et al. (35), Mahmoudi Rad et al. (36), and Asadi et al. (37). There is a consequential association between infection and use of contraceptive pills. In this investigation, 40% of patients had also used contraceptive pills. Yusuf et al. (38) revealed a meaningful association between use of contraceptives and the prevalence of vaginal Candida infection. They disclosed among all contraceptives, use of oral contraceptive pills (OCP) was the most common cause of vaginitis compared to injectable one. We reported 54.6% of VVC, which is higher than reported by Dharmik et al. (39), Dou et al. (40), Mukasa et al. (41), Masri et al. (25), and Hedayati et al. (35) who reported 18.4% in India, 51.4% in China, 45.3% in Uganda, 17.2% in Malaysia, and 17.2% in Mazandaran, Iran, respectively. Candida albicans was the most prevalent species among patients, in accordance with previous studies from various countries (21, 42-44). Some studies have shown an increase of non-albicans Candida species in volvuvaginal specimens (45, 46), this may be related to the prolonged use of antifungal agents, incomplete therapeutic regimens, and self-prescribed antifungal therapy (47). Furthermore, non-albicans Candida species such as Candida glabrata and Candida krusei make a poor response to azole agents such as fluconazole, which can be a reason for increasing non-albicans Candida species among patients with volvuvaginal candidiasis.
4.1. Conclusion
Emerging of non-albicans Candida species among clinical specimens and high resistance of Candida species to various antifungal agents are crucial concerns in medical mycology. For better management of fungal infections like VVC, precise identification of clinical isolates and a good choice of antifungal agent after antifungal susceptibility testing are inevitable. Since VVC is a prevalent, recurrent, and bothersome fungal infection, especially in pregnant women, the controlling of predisposing factors and personal hygiene are extremely recommended among vulnerable population.