1. Background
Staphylococcus epidermidis is considered as part of the body’s normal flora for a long time without any pathogenic significance. Nowadays, S. epidermidis is identified as one of the bacterial causes of opportunistic pathogen in implant-associated and nosocomial infections and regarded as one of the top nosocomial infection causes (1, 2). Quorum-sensing systems are considered as important mechanisms for pathogenesis and bacterial communication (3, 4). The accessory gene regulator (agr) system is one of the best known quorum-sensing systems in staphylococci (4, 5). The agr quorum-sensing system regulates certain bacterial activities, such as biofilm formation, symbiosis, virulence, competence, conjugation, antibiotic production, motility, and sporulation (6-8). The agr system, which is generally conserved in staphylococci, encoded by agr locus, comprises of two transcripts, called RNAII and RNAIII (9-11). There are three different agr types in clinical isolates of S. epidermidis (6). Some reports have suggested a correlation between agr groups and infection (12).
The most fundamental concept of epidemiological typing is whether the isolates, which are isolated from infections, belong to a chain of transmissions and correlate with each other, in other words, whether they have a common ancestor (13). Genotypic and phenotypic diversity have been created due to point mutation, recombination or other addition methods as well as deletion of mobile genetic elements (14). Different methods of typing and criteria are used to determine the closeness of strains, although time and place should also be considered (13-16).
Staphylococcus epidermidis is one of the most important causes of catheter-related nosocomial infections. About 70% of strains isolated from the hospital environment are methicillin-resistant and most of them are resistant to almost all antibiotics. The ability of quick and accurate recognition of the relationships between clinical isolates is critical to study possible outbreaks and cross-transmission of bacteria. The results of different molecular methods on Staphylococcus epidermidis suggest a considerable genetic diversity. Despite the observable variability, these methods are able to differentiate the strains in different patients, wards, or hospitals, and are considered valuable tools for controlling infections caused by Staphylococcus epidermidis (17). Multiple-Locus VNTR analysis (MLVA) is designed based on the number of replicates of short repetitive sequences in the target locus of the bacterial genome and based on polymerase chain reaction by a simple method and at a low cost, and provides numerical data of the number of repetitions in a locus. The number of replicate copies varies among different strains. These sequences are known as VNTR, and the analysis of the number of VNTR is called Multiple-Locus VNTR analysis (MLVA). This method is reliable, rapid and low-cost, and can provide transferable information in the form of codes that can be saved in databases and exchanged between different laboratories. Working with the genetic material of bacteria does not have the risk of exposure to live microorganisms and is one of the advantages of this method (2). The MLVA is a method for typing bacteria, which has been applied to several different species of bacteria. This method has appropriate differentiation power, is low cost, does not take long to run, and can be performed for a large number of samples (18-25).
2. Objectives
This study aimed at determining the diversity of Staphylococcus epidermidis accessory gene regulator and clonality in clinical isolates from intensive care unit patients of Tehran, Iran.
3. Methods
3.1. Bacterial Isolates
A total of 59 Staphylococcus epidermidis isolates were obtained from intensive care unit patients of a teaching hospital, during year 2013, Tehran, Iran, in a cross-sectional study. Strains were isolated from blood (n = 50), urine (n = 4), tracheal (n = 4), and wound (n = 1) samples. In order to identify S.epidermidis, conventional bacteriological tests were performed. At first, the sample was cultured on sheep blood agar (Merck, Germany) under aerobic conditions and overnight at 37°C; suspected colonies were identified by performing Gram staining, catalase test, growth on mannitol salt agar (Merck, Germany), without mannitol fermentation, tube coagulase test with rabbit plasma, ability of urease production, D-manose and D-maltose fermentation, and no fermentation of D-trehalose (26, 27).
3.2. DNA Extraction
DNA of each S. epidermidis isolate was extracted from 1 mL of overnight bacterial culture. Extraction was performed by adding 10 mmol/L tris and 1 mmol/L ethylene diamine tetraacetic acid (pH = 8) buffer and lysostaphin, at 95°C for five minutes, then 0°C for five minutes, and centrifuged in 10000 r/minute for five minutes. The supernatant was used as the template DNA in polymerase chain reaction (PCR). DNA was measured using a BioPhotometer (Eppendorf, Germany) to determine the concentration and purity (2, 28, 29).
3.3. Multi Locus VNTR Analysis (MLVA) and agr Typing
The MLVA was performed for S. epidermidis isolates, using seven VNTR loci, including SE2395, SE0331, SE 828, SE1632, SE0175, Se2, and Se4, as previously described (16, 17). Polymerase chain reaction was performed for each locus in a 25-μL volume PCR reaction containing specific primers (Pishgam biotech, Iran). The loci, tandem repeat sizes, and primers are listed in Table 1.
| VNTR Locus | Sequence (5→3) | PCR Condition | Left Flanking Sequence | Right Flanking Sequence | Tandem Size | Expected Size of Strain ATCC 12228, bp | Reference |
|---|---|---|---|---|---|---|---|
| SE2395 (Fibrinogen binding protein) | Forward: CAGGCCATATAGACCTGGCTTG | 2 min, 94°C; 35 cycles of: 15 s, 94°C; 20 s, 60°C; 70 s, 72°C; final extension, 10 min, 72°C | 140 | 499 | 18 | 1625 | (17) |
| Reverse: TGCTGATGGGGAAGATGTTCGTG | |||||||
| SE0331 (sdrG protein) | Forward: ATGGGGAAGAAGTCCATGTA | 2 min, 94°C; 35 cycles of: 15 s, 94°C; 20 s, 55°C; 60 s, 72°C; final extension, 10 min, 72°C | 72 | 59 | 18 | 671 | (17) |
| Reverse: CATTAGCTCCTGTATCCGGT | |||||||
| SE 828 (cell wall surface protein) | Forward: TGCCACTGGTAATCAAAATG | 2 min, 94°C; 35 cycles of: 15 s, 94°C; 20 s, 55°C; 60 s, 72°C; final extension, 10 min, 72°C | 134 | 20 | 273 | 815 | (17) |
| Reverse: GCATCTTTATCTGTACCGCC | |||||||
| SE1632 (sdrH protein) | Forward: ATGACACTAGTCGCACAGGA | 2 min, 94°C; 35 cycles of: 15 s, 94°C; 20 s, 55°C; 60 s, 72°C; final extension, 10 min, 72°C | 94 | 34 | 18 | 468 | (17) |
| Reverse: CGGTATGTGAACCCTTACCT | |||||||
| SE0175 (aap) | Forward: TGAAGCACCACAGATGTCTTCTAC | 2 min, 94°C; 35 cycles of: 15 s, 94°C; 20 s, 55°C; 60 s, 72°C; final extension, 10 min, 72°C | 31 | 61 | 48 | 129 | (17) |
| Reverse: GGGCTTCTGAAAATTGTGTT | |||||||
| Se2 | Forward: AGGCCCAAATAAAAAGCAAA | 10 min, 95°C; 30 cycles of: 1 min, 95°C; 1 min, 55°C; 1 min72°C; final extension, 5 min, 72°C | 221 | 61 | 58 | 524 | (16) |
| Reverse: AACTGACGCTCCAGGAGAAG | |||||||
| Se4 (PTS enzyme II) | Forward: TTCATTGTCCCCTGTCTTCT | 10 min, 95°C; 30 cycles of: 1 min, 95°C; 1 min, 55°C; 1 min72°C; final extension, 5 min, 72°C | 71 | 83 | 57 | 381 | (16) |
| Reverse: TCGATCCTGGTAAAGCGATTA |
Loci, Tandem Repeat Sizes, and Primers for Multi Locus VNTR Analysis
Specific primers were used for agr diversity determination (30). DNA amplification was performed in a 25-μL PCR reaction. Multiplex PCR conditions were as follows: five minutes, 95°C; 30 cycles of one minute at 94°C; one minute at 55°C; one minute at 72°C; and final extension, 10 minutes at 72°C. Specific primers for different types of agr are shown in Table 2.
| agr Type | Sequence (5→3) | Expected Size, bp | Reference |
|---|---|---|---|
| Type I | Forward: GGCATTAGTCGGATTAATTATTACG | 438 | (30) |
| Reverse: TGTAGGCCTGCAAACGG | |||
| Type II | Forward: TTTACCATTTGCAGCTATACAAGTG | 575 | (30) |
| Reverse: ATAACAATAATATAACCAAACTCAAAAGTACAG | |||
| Type III | Forward: GAAAGAGTGTATTCAATGGATGAGC | 338 | (30) |
| Reverse: TAAATATTATGTATTATATCTTCAGTATATAAAGAGATGA |
Specific Primers for Different Types of agr
Amplification was performed in a MJ mini Gradient thermal cycler PTC-1148, USA. The amplified products were visualized by UV light after electrophoresis on 1% agarose gel. A positive control (S. epidermidis ATCC 12228) and negative control (reaction mixture without DNA) were included in each PCR run.
3.4. Data Analysis
The number of repeats and their length were calculated as follows: each amplicon was read, and the size of flanking regions was subtracted from the amplicon size, and then divided by Tandem size. The number of repetitions of each tandem was categorized and analyzed, unweighted pair group method with arithmetic averages (UPGMA) dendrogram, and minimum spanning tree (MST), were constructed as previously described (31-33). The MLVA plus online software was used for the MLVA typing data analysis.
4. Results
Of the 59 Staphylococcus epidermidis isolates obtained from intensive care unit patients, agr type I was observed in 29 (49%), while each of the agr type II and agr type III were observed in 10 (17%) cases. Ten (17%) of the isolates were untypeable with using primers.
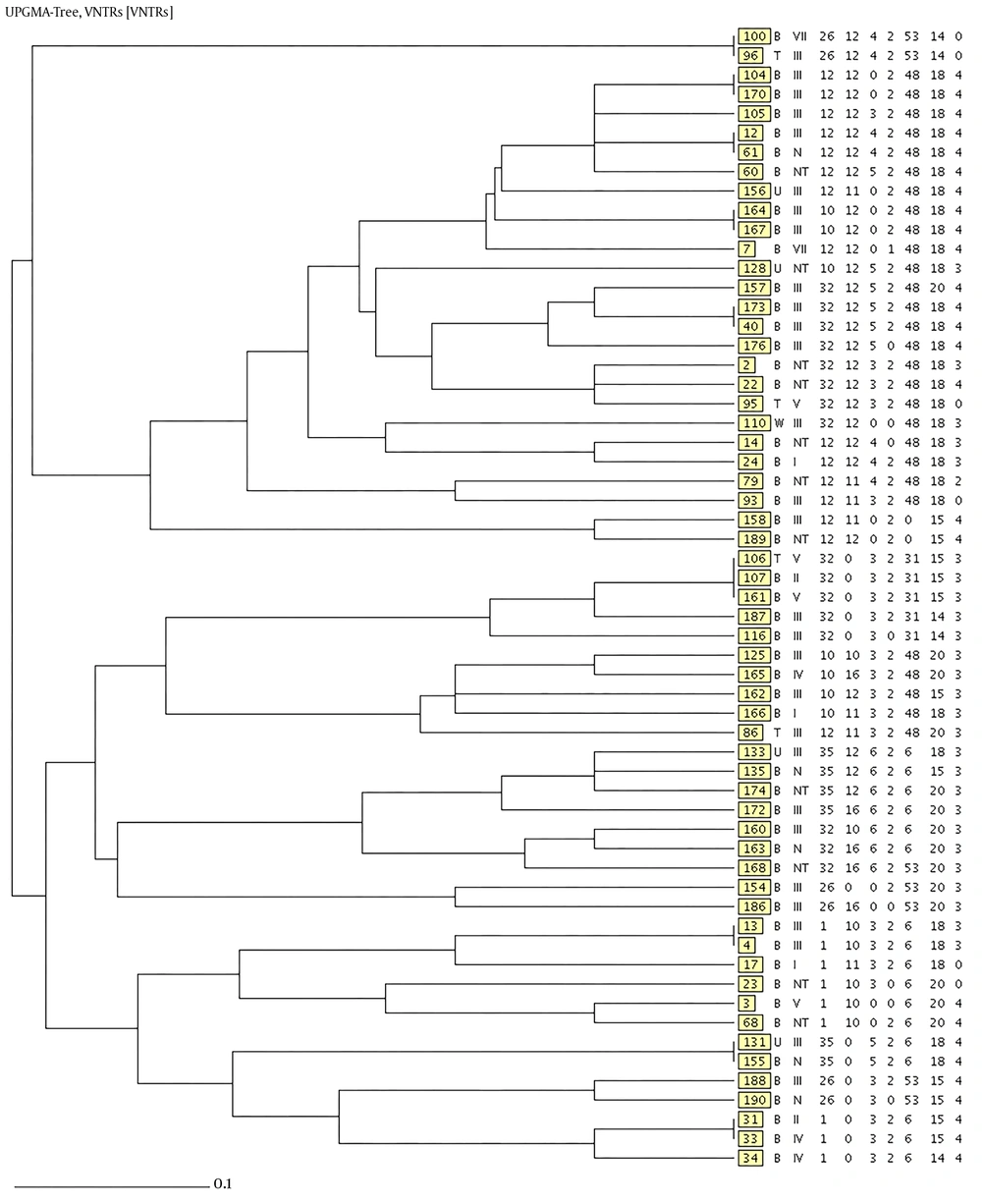
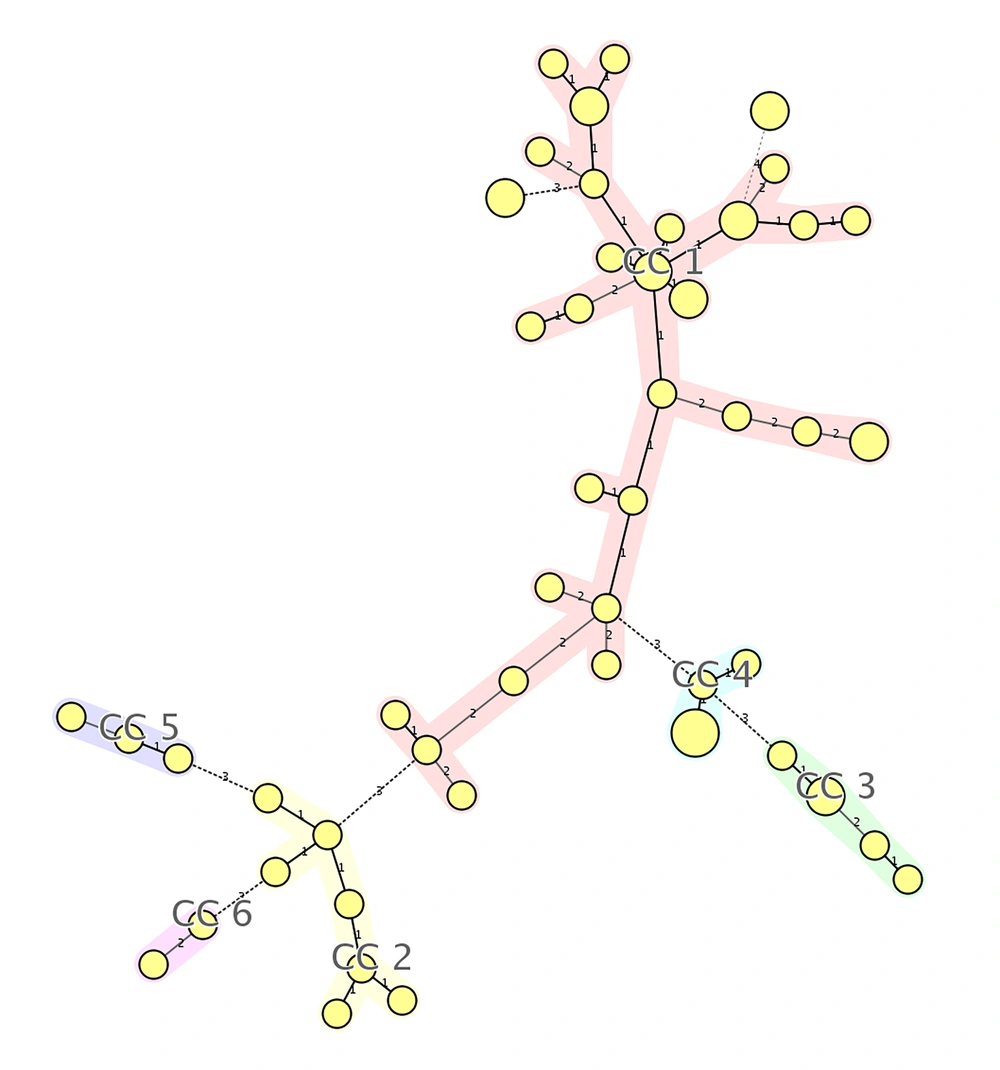
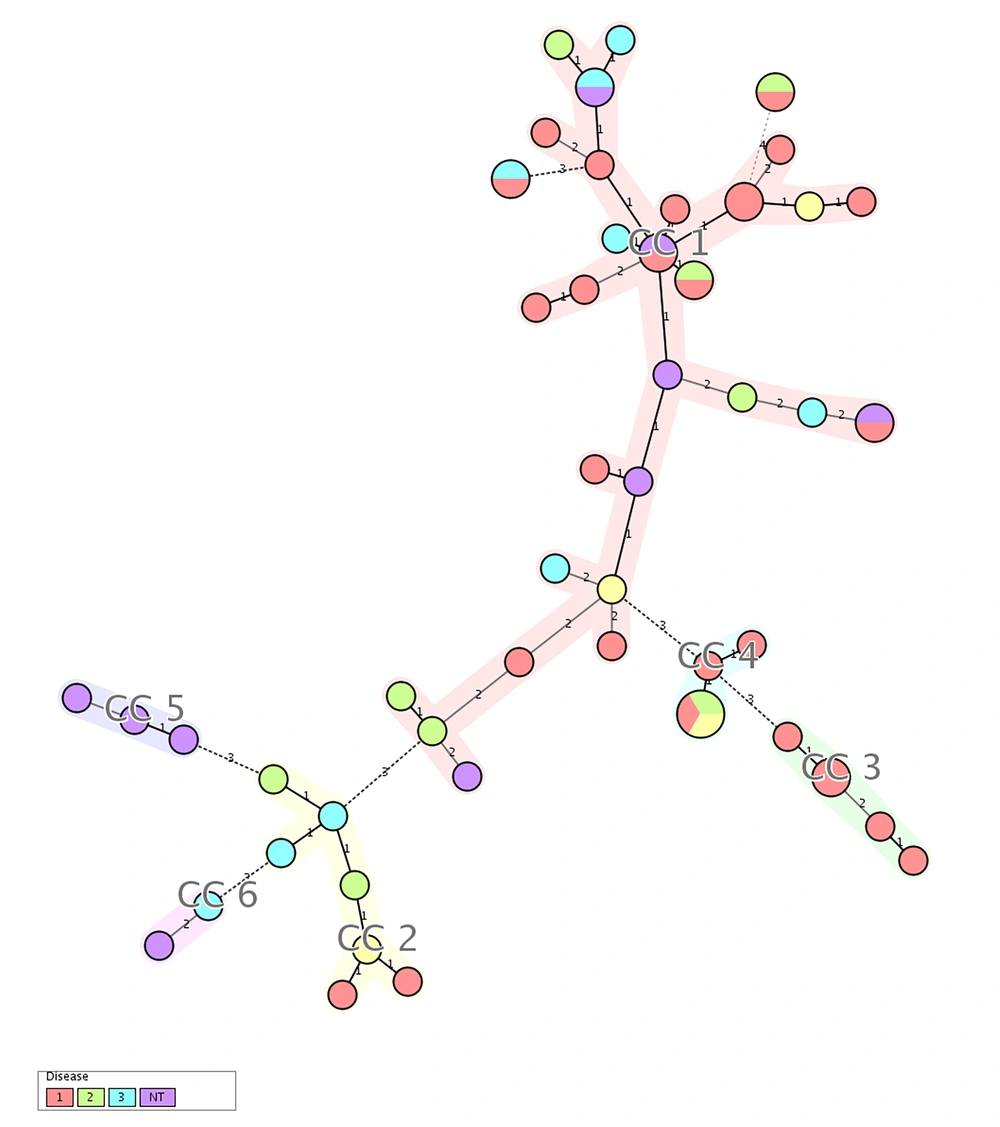
The MLVA was performed for seven VNTR loci; a total of 49 MLVA genotypes were discriminated. Isolates were classified to six clonal complexes. Of the 59 isolates, 33 were included in clonal complex 1 (CC1), which was the largest, and among them, 15 (45.4%) harbored agr type I, five (15.2%) agr type II, five (15.2%) agr type III, and six (18.2%) were recognized as non-typeable. CC2, CC3, CC4, CC5, and CC6 included seven, five, five, three, and isolates, respectively. In CC3, all of isolates were categorized as agr type I, while all of isolates in CC5 were classified as non-typeable. Four isolates were classified as singleton, which were different in two or more loci and unrelated to other clonal complexes. The unweighted pair group method with arithmetic averages (UPGMA) dendrogram is shown in Figure 1, the minimum spanning tree (MST) is shown in Figure 2, and the correlation of clonal complexes and agr types is shown in Figure 3.
Minimum spanning tree (MST) representation. Each MLVA profile is shown by a circle, small circle presents one, medium circle presents two, and large circle presents three isolates. The number of loci, which differed between two MLVA profiles is indicated on the lines connecting the MLVA profiles.
5. Discussion
This research attempted to determine agr diversity and genetic relatedness of 59 Staphylococcus epidermidis isolates obtained from intensive care unit patients of Tehran, Iran. In this study, agr type I was the most frequent agr type (49%). This amount was reported in the United State of America (34) and China (35) as 89% and 68.2%, respectively, which was significantly higher than the present study. In France the frequency of type I agr in Staphylococcusepidermidis was reported as 48.5% by Lina et al. (30), which was not significantly different to the findings of the current study. agr type II was found in ten (17%) of the investigated isolates, and did not show significant differences with 11% and 19.3% reported in the United State of America (34) and China (35), respectively. However, it was significantly lower than 31.8% in France (30). agr type III was found in ten (17%) of this study isolates, which was significantly lower than 49% in France (30), higher than 0% reported in United State of America (34), and had no difference with 12.5% reported in China (35). In this study, ten isolates were not typeable using primers, which was significantly higher than 0% and 2% reported in United State of America (34) and France (30), respectively.
Using MLVA in 59 isolates, 49 different genotypes and six clonal complexes were identified. The CC1 included 33 isolates that comprised 56% of the isolates. This clonal complex may indicate the establishment of Staphylococcus epidermidis at the intensive care unit (ICU). Given that the samples were collected during the interval of about one year, a little variation in this clonal complex was not unexpected. Several other clonal complexes with a small number of isolates in each could indicate the entry of a new bacteria in the ward and its settlement. The isolates, identified as singleton, are possibly bacteria that have entered the ward by the patient, visitors or medical personnel. Four isolates were also identified as singleton. In Sweden, during years 2001 to 2002, MLVA was conducted for 30 clinical strains of Staphylococcus epidermidis, isolated from different nosocomial infections, and 16 different genotypes were identified. The strains were very close to each other and were disseminated in different hospital wards (16). A total of 96 strains of Staphylococcus epidermidis as part of three collections were studied, including 21 isolates collected from infants in Norway during years 2005 to 2007, 49 strains isolated from 13 patients in Switzerland during years 2002 to 2005, and 26 strains isolated from 12 patients in the USA during years 1990 to 1993. No close relationship was observed between different groups, and similar profiles were not observed in each group either, indicating genetic diversity of isolates in each group (17). In 89 isolates of Staphylococcus epidermidis isolated from nasal swabs of people in an almost separate region in France with an interval of 16 months in October 2006 and June 2008, 62 MLVA profiles were observed that were not associated with the sample isolation time and SCCmec type. Of 45 profiles, each was observed only in one sample (36). The MLVA revealed heterogeneous profiles and high variability in 58 strains of Staphylococcus epidermidis isolated from nasal samples of orthopedic surgery patients in a hospital in Paris, during year 2005. No relationship was observed between MLVA profile and the SCCmec type (37).
Given that the strains of the present study were collected from the ICU, the results of this study are similar to a study conducted in Sweden (16), while they are different from other studies (17, 36, 37), in which nasal isolates or isolates with different sources were investigated.
5.1. Conclusion
The MLVA results of this study suggest that big clone isolates were settled at the ICU, while small clone isolates were settled, and singleton isolates entered the ward by visitors or medical personnel and caused infection, also agr type I was observed in the majority of the isolates, which is in accordance with other similar studies.