1. Context
Urinary tract infection (UTI) is the most common bacterial infection with the high economic burden that affects a large number of populations. The range of clinical symptoms of the infection is different from asymptomatic bacteriuria to bladder inflammation, pyelonephritis, septic shock, and defects in several organs (1, 2). Both hospital-acquired and community-acquired urinary tract infections can lead to serious complications such as urinary tract disorders, uremia, hypertension, and even death (3). It is the most common infection among patients admitted to hospitals and laboratories. It can be observed in all age groups and in both genders although it is more likely to occur among young women (4). Studies have indicated that about 50% of women and 12% of men suffer from urinary tract infection throughout their life (5). Moreover, 20 - 30% of women experience a recurrence of this infection during 6 - 12 months (5, 6). Therefore, this is a major public health problem (7). Throughout the world, about 150 million people are diagnosed with a UTI each year (8). In most cases, bacterial agents, especially Gram-negative bacteria such as E. coli, are the cause of this infection. Other Gram-negative bacteria include Klebsiella, Proteus, Pseudomonas, Serratia, and Enterobacter, which are involved in a small percentage of urinary tract infections (4, 9). Isolated urinary pathogens vary depending on age, gender, catheterization, hospitalization, and previous use of antibiotics (10). Due to the acquisition of antibiotic resistance genes by the bacteria over time in different geographical areas, on the one hand, and the change in the susceptibility patterns of bacteria to antibiotics, on the other hand, the selection of the appropriate antibiotics for treatment has become a challenge and it is more based on the information obtained from determining the pattern of antimicrobial resistance in the given area (3).
Cotrimoxazole is made from a combination of two antibiotics including trimethoprim and sulfamethoxazole, both of which disrupt folic acid metabolism in bacteria through different mechanisms. Moreover, it is the first antibiotic prescribed for the treatment of urinary tract infection (11). Folic acid producing bacteria are sensitive to this medicine; therefore, the drug stops their proliferation (12-14).
The widespread use of this drug due to the low cost, fewer side effects, and availability have led to increased resistance to cotrimoxazole among urinary pathogens (15).
The treatment of urinary tract infections can be often done on an experimental basis. An important principle in the treatment of such infections is the use of the results of the bacterial resistance patterns causing the infection. Furthermore, because of the continuous rise of antibiotic resistance, the regular monitoring of antibiotic resistance is necessary for improving the empirical antibiotic treatment guidelines (16). According to numerous studies conducted on the prevalence of resistance to cotrimoxazole among the urinary pathogenic bacteria, a meta-analysis seemed necessary in order to validate the results of these studies.
2. Evidence Acquisition
2.1. Search Strategy
A systematic review and meta-analysis was conducted in Persian and English by searching the national and international databases including SID, Magiran, IranDoc, IranMedex, MedLib, PubMed, ISI, Web of Science, Scopus, and Google Scholar to find studies published from 1992 to 2015 concerning the patterns of drug resistance to cotrimoxazole in microbial strains isolated from urinary tract infections. These databases were investigated using keywords in Persian (urinary tract infections, Gram-negative bacteria, Gram-positive bacteria, cotrimoxazole, and antibiotic resistance) and their English equivalent, with all the possible combinations including important, original, and sensitive words. In addition, the reference list of the selected articles was used to find additional resources.
2.2. Study Selection
All group or cross-sectional studies were considered among those reporting the prevalence of bacterial resistance to cotrimoxazole in urinary tract infections. Two researchers (S-Gh, R-P) independently took the responsibility for selecting the articles and assessing their validity. Any discrepancy between the two raters was resolved by a third reviewer (I-P). The current study applied a blinding and task separation to the study selection. The inter-rater reliability was calculated based on kappa statistics, which was obtained at 85%.
The selection of papers for the study was completed based on three stages including title, abstract, and full text. Studies with insufficient information, non-cross-sectional designs, studies in conjunction with other non-UTI infections, review studies, abstracts of conference proceedings, published papers in languages other than English and Persian, meta-analyses, systematic, and repetitive studies were excluded from the analysis.
2.3. Data Extraction and Quality Assessment
First, the title of all articles was reviewed for screening the eligibility criteria. Every paper, which was inconsistent with the eligibility criteria, was excluded. Afterward, the abstracts were checked and then, the full text of articles was assessed to identify the inclusion criteria. Those articles that did not satisfy the inclusion criteria nor had a weak correlation with the objectives of the study were excluded. Then, all the articles included in the final stage of analysis were assessed by using checklists to extract the following data: first author, year of publication, year of study, study location, sample size, mean age, gender, type of isolation, prevalence of urinary pathogens, bacterial pathogens resistant to cotrimoxazole, and standard methods for determining antibiotic susceptibility to antibiotics (Table 1).
| First Author (Reference) | Publication Year | Study Location | Sample Size | The Prevalence of Pathogens and Their Drug Resistance to Cotrimoxazole | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli (%) | Klebsiella (%) | Staphylococcus (%) | ||||||||||
| Prevalence | Resistance | Susceptibility | Prevalence | Resistance | Susceptibility | Prevalence | Resistance | Susceptibility | ||||
| Sharifi (16) | 2014 | Yasouj | 120 | - | 62.5 | 37.5 | - | - | - | - | - | - |
| Molazadeh (17) | 2012 | Gaharbi Azerbaijan | 1900 | 39.3 | 60.3 | 29.8 | - | - | - | - | - | - |
| Mohajeri (18) | 2011 | Kermanshah | 1114 | 75 | 58.7 | 36.7 | - | - | - | - | - | - |
| Molazadeh (17) | 2012 | Fasa | 2484 | 76.3 | 59.4 | 40.6 | 16.6 | 51.7 | 48.3 | - | - | - |
| Mosavian (19) | 2004 | Ahvaz | 38 | 34 | 35.3 | 64.7 | 20 | - | - | 14 | 33.3 | 50 |
| Madani (20) | 2008 | Kermanshah | 1815 | 45.4 | 61.1 | 27.7 | - | - | - | - | - | - |
| Sorkhi (21) | 2005 | Babol | 188 | 59.5 | 81 | 19 | - | - | - | - | - | - |
| Ghutaslou (22) | 2005 | Tabriz | 2013 | 79 | 64.7 | 35.3 | 10.3 | - | - | - | - | - |
| Nourozi (23) | 2000 | Tehran | 313 | 53.5 | 78 | 22 | 4.6 | - | - | 3.2 | - | - |
| Alaei (24) | 2008 | Tehran | 510 | 72 | 66 | 34 | 9 | - | - | 6.1 | - | - |
| Borji (25) | 2001 | Zahedan | 429 | - | 80.5 | 16.7 | - | - | - | - | - | - |
| Torabi (26) | 2007 | Zanjan | 118 | 48.4 | 57.4 | 42.6 | 17.3 | - | - | 15.9 | 71.1 | 28.9 |
| Hajizade (27) | 2003 | Tehran | 150 | 50 | 72 | 28 | 10 | 70 | 30 | 19 | 55 | 45 |
| Eghbalian (28) | 2005 | Hamedan | 156 | 82.1 | 34.4 | 65.6 | 2.6 | - | - | 5.1 | - | - |
| Barati (29) | 2011 | Kolaleh | 360 | - | 47 | 48.1 | - | - | - | - | - | - |
| Farshad (30) | 2009 | Jahrom | 90 | - | 76 | 24 | - | - | - | - | - | - |
| Jarsiah (4) | 2014 | Tehran | 208 | 73.1 | 65.5 | 25.4 | 6.7 | - | - | - | - | - |
| Mirmostafa (31) | 2013 | Karaj | 100 | 83 | 59 | 41 | 3 | - | - | - | - | - |
| Tajvidi (32) | 2014 | Tehran | 372 | - | 51 | 47 | - | - | - | - | - | - |
| Asgharian (33) | 2011 | Tenkabon | 357 | 84.6 | 83 | 17 | - | - | - | - | - | - |
| Sharif (34) | 1999 | Kashan | 157 | 52 | 56 | 44 | 14.7 | 57.8 | 42.2 | - | - | - |
| Rahimi (35) | 2014 | Isfahan | 301 | 64.5 | 70 | 29 | 7.3 | 31 | 69 | 11.3 | - | - |
| Ranjbaran (36) | 2013 | Arak | 50 | - | 88 | 12 | - | 70 | 30 | - | - | - |
| Mokhtarian (37) | 2006 | Gokabad | 353 | 66 | 62.2 | 16.7 | 7.9 | - | - | 21.8 | - | - |
| Beheshti (38) | 2005 | Tabriz | 103 | - | 76.1 | 28.4 | - | - | - | - | - | - |
| Assefzadeh (39) | 2009 | Ghazvin | 224 | 61.2 | 61.5 | 38.5 | 8.9 | 64.3 | 35.7 | 7.2 | 45.5 | 54.5 |
| Tarhani (40) | 2003 | Khoramabad | 127 | 73.2 | 54.3 | 45.7 | 49.4 | 7.1 | 92.9 | - | - | - |
| Hamid-Farahani (41) | 2012 | Tehran | 456 | 60.3 | 37 | 61.5 | 13 | - | - | 22.1 | - | - |
| Mohammadi (42) | 2006 | Falavarjan | 209 | 54.1 | 63.4 | 35.9 | 15.8 | - | - | 17.2 | - | - |
| Langarizadeh (43) | 2010 | Tabriz | 72 | - | - | - | - | 95.8 | 2.8 | - | - | - |
| Fahimi (44) | 2004 | Tehran | 170 | 61.1 | 75.3 | 19.4 | 2.9 | 2.8 | - | - | ||
| Babaie Kasmaei (45) | 2012 | Tehran | 123 | - | 93.3 | 6.7 | - | - | - | - | - | - |
| Arbab Soleimani (46) | 2014 | Semnan | 100 | 70 | 42 | 58 | - | - | - | - | - | - |
| Tashkori (47) | 2011 | Rafsanjan | 146 | - | 62.3 | 37.7 | - | - | - | - | - | |
| Moulana (48) | 2013 | Babol | 770 | 48.6 | 69.3 | 30.7 | 17.9 | - | - | 8 | - | - |
| Heidari- Soureshojani (49) | 2013 | Chaharmahal va Bakhtiari | 74 | 70.3 | 42.4 | 51.5 | - | - | - | 20.3 | - | - |
| Soltan Dallal (50) | 2011 | Tabriz | 400 | 47 | 65 | 35 | - | - | - | - | - | - |
| Sharifi Yazdi (51) | 2013 | Tehran | 300 | - | - | - | - | - | - | 77.7 | 65.1 | 43.9 |
| Fesharakinia (52) | 2012 | Birjand | 100 | 75 | 69.9 | 20.5 | - | - | - | - | - | - |
| Jalalpour (53) | 2009 | Isfahan | 91 | 84.6 | 59.2 | 40.8 | 15.4 | 50 | 50 | - | - | - |
| Fallah (54) | 2012 | Tehran | 200 | - | 67.7 | 32.3 | - | - | - | - | - | - |
| Khodadoost (55) | 2013 | Kermanshah | 140 | - | 62.1 | 37.3 | - | - | - | - | - | - |
| Ghadiri (56) | 2009 | Kermanshah | 87 | - | 72.4 | 19.5 | - | - | - | - | - | - |
| Sahebnasagh (57) | 2015 | Tehran | 1123 | 50 | 61.4 | 38.6 | - | - | - | - | - | - |
| Safkhani (58) | 2014 | Tehran | 136 | 51.5 | 60 | 37.3 | 11.8 | 43.8 | 56.2 | 13.2 | - | - |
| Barari Sawadkouhi (59) | 2013 | Babol | 128 | 89 | 62.3 | 37.7 | 3.1 | 50 | 50 | - | 0 | 100 |
| Akya (60) | 2015 | Kermanshah | 200 | - | 57.5 | 42.5 | - | - | - | - | - | - |
| Dezfolimanesh (61) | 2013 | Kermanshah | 100 | - | 55.6 | 34.9 | - | - | - | - | - | - |
| Isvand (62) | 2014 | Dezfoul | 160 | 63.3 | 11.2 | 88.8 | 20.6 | - | - | 4.4 | - | |
| Esmaeli (63) | 2013 | Hamedan | 141 | 61 | 28 | 73 | - | - | - | 8.5 | 66.7 | 33.3 |
| Sedighi (64) | 2014 | Hamedan | 50 | - | 70 | 28 | - | - | - | - | - | - |
| Hosseini (65) | 2014 | Ghazvin | 1204 | 1.61 | 48 | 52 | 10.6 | 43 | 57 | - | - | - |
| Bagheri (66) | 2014 | Gorgan | 111 | - | - | - | 40.5 | 1.31 | 9.68 | - | - | - |
| Molazadeh (17) | 2012 | Fasa | 283 | 64.3 | 5.66 | 26.7 | 5.14 | 1.47 | 1.44 | 4.6 | 9.42 | 7.35 |
| Molazadeh (17) | 2012 | Fasa | 2484 | 64.7 | 7.68 | 31.3 | 9.23 | 4.60 | 6.39 | - | - | - |
| Molaabaszadeh (67) | 2013 | Tabriz | 5701 | 58.4 | 9.63 | 33 | - | - | - | - | - | - |
| Ahangarkani (68) | 2015 | Babol | 128 | 78 | 67 | 22 | - | - | - | - | - | - |
| Neamati (69) | 2015 | Kashan | 150 | - | 7.64 | 7.32 | - | - | - | - | - | - |
| Sedighi (64) | 2014 | Hamedan | 100 | - | 70 | 26 | - | - | - | - | - | - |
| Nasiri Moghadam (70) | 2000 | Birjand | 111 | 65 | 65 | 35 | - | - | - | - | - | - |
| Savadkoohi (71) | 2007 | Babol | 160 | 70 | 67 | 33 | - | - | - | 6.5 | 7.66 | 3.33 |
| Emam Ghoreishi (72) | 2014 | Jahrom | 108 | - | 4.71 | 6.28 | - | - | - | - | - | - |
| Esmaeili (73) | 2005 | Mashhad | 166 | 1.74 | 8.75 | 2.24 | 2.7 | 3.33 | 7.66 | 3.4 | - | - |
| Sedighi (74) | 2010 | Hamedan | 100 | - | 70 | 26 | - | - | - | - | - | - |
| Zamanzad (75) | 2005 | Shahrkord | 100 | 42 | 2.76 | 8.23 | 38 | 7.84 | 3.15 | 12 | - | - |
| Zamanzad (75) | 2005 | Shahrkord | 100 | 58 | 5.65 | 5.34 | 16 | 75 | 25 | 7 | - | - |
| Norouzi (76) | 2006 | Jahrom | 356 | 80.3 | 51 | 1.41 | 10.7 | - | - | 84 | - | - |
| Nakhaie Moghadam (77) | 2010 | Mashhad | 109 | - | 1.55 | 9.44 | - | - | - | - | - | - |
General Specifications and Data of Articles Reviewed in the Meta-Analysis
To evaluate the quality of the studies, seven items on the STROBE (strengthening the reporting of observational studies in epidemiology) checklist were considered: meticulous references based on the framework of the study, setting of the study, inclusion and exclusion criteria, definition of UTI and criteria, identification and measurement methods of UTI and criteria, reporting the main outcome, the number of study population, and the target population of the study.
The studies, which adhered to all seven items on the STROBE checklist, were labeled as high quality while those observing six items were of medium quality. The studies that did not meet two or more items on the STROBE checklist were classified as low-quality studies.
2.4. Statistical Analysis
Given that in each article, the prevalence of antibiotic resistance and the number of samples were extracted, a binomial distribution was used to calculate the variance of each study and average weight was applied to combine the prevalence of different studies. A weight was given to every study inversely proportional to its variance. According to a large difference in prevalence rates between different studies (heterogeneity of studies) and based on the significant heterogeneity index (I2 = 95%), a random-effects model was used in the meta-analysis. A funnel plot was used to detect the publication bias graphically. It is a bivariate scatter plot (x, y) of the study sample size versus the study estimate of treatment difference or effect size. There is a formal test for publication bias based on the linear regression analysis. It includes both intercept and slope parameters given as yi = α + βxi + εi, for i = 1, ..., r, where r is the number of studies, yi is the ‘standardized estimate’, xi is the ‘precision’ of studies and εi the error term.
3. Results
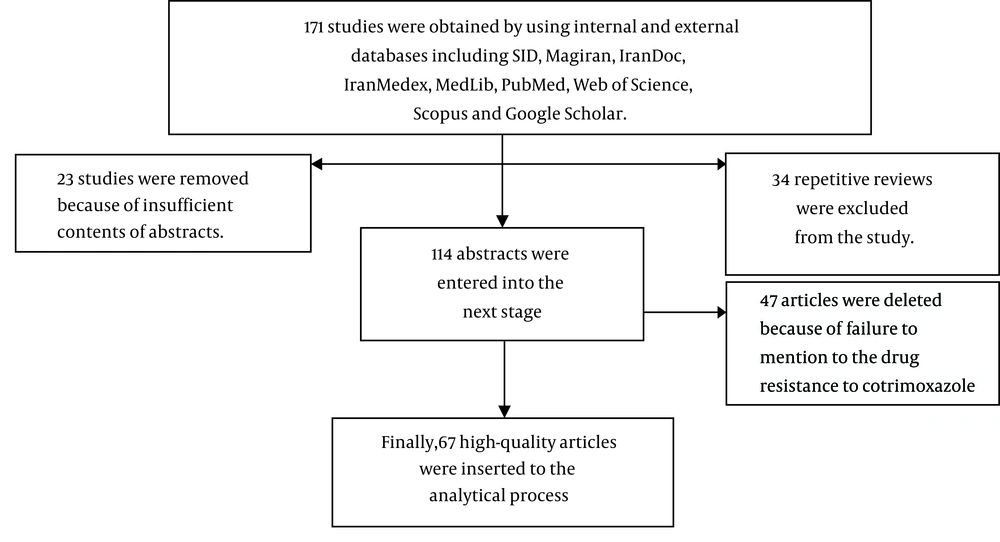
In the initial search, 171 full papers and abstracts were obtained. In the next phase, 23 papers and abstracts were removed because of mismatch and insufficient contents of abstracts. Then, 34 other repetitive reviews were excluded from the study. After evaluation of articles, 47 ones were deleted because of failure to mention the drug resistance to cotrimoxazole and finally, after a thorough review of the selected articles, 67 studies, which were published between 1995 and 2015, entered into the meta-analysis (Figure 1). Based on the quality assessment, 42 studies were classified as high quality and 25 as moderate quality. General specifications and data from articles are shown in Table 1.
In all the studies included in this meta-analysis, the middle urine samples were cultured on the appropriate media and the bacteria were identified by standard methods. The results obtained from these studies showed that 29399 cases among the total number of samples had a urinary tract infection, 78.40% of which were related to women while 21.60% of these samples were found in men and the highest proportion (59.40%) was associated with outpatients. The most common bacteria that caused urinary tract infection and their prevalence are indicated in Table 2. As can be seen, the most common pathogen responsible for urinary tract infection was E. coli with the frequency of 64% (Figure 2). Klebsiella with a frequency of 12%, Staphylococcus with a frequency of 10%, and Enterobacter with a frequency of 6% were in the next ranks. In addition, other bacteria responsible for urinary tract infection included Enterococci, Citrobacter, Acinetobacter, Pseudomonas, Proteus, and Enterobacter with an overall frequency of 8% that had marginal roles in the development of urinary tract infections (Table 2).
| Bacteria Type | The Number of Studies | Prevalence | Confidence Interval (CI%95) | Heterogeneity Index I2 (%) | P-Value |
|---|---|---|---|---|---|
| E. coli | 45 | 64% | 68 - 60 | 97.6 | < 0.001 |
| Klebsiella | 31 | 12% | 9 - 15 | 96.6 | < 0.001 |
| Staphylococcus | 24 | 10% | 7 - 12 | 93.3 | < 0.001 |
| Enterobacter | 23 | 6% | 5 - 7 | 88.9 | < 0.001 |
| Other species | 34 | 8% | 2 - 12 | 59.2 | < 0.001 |
The Frequency Distribution of Bacterial Agents of Urinary Tract Infection in Terms of the Number of Studies Included in the Meta-Analysis
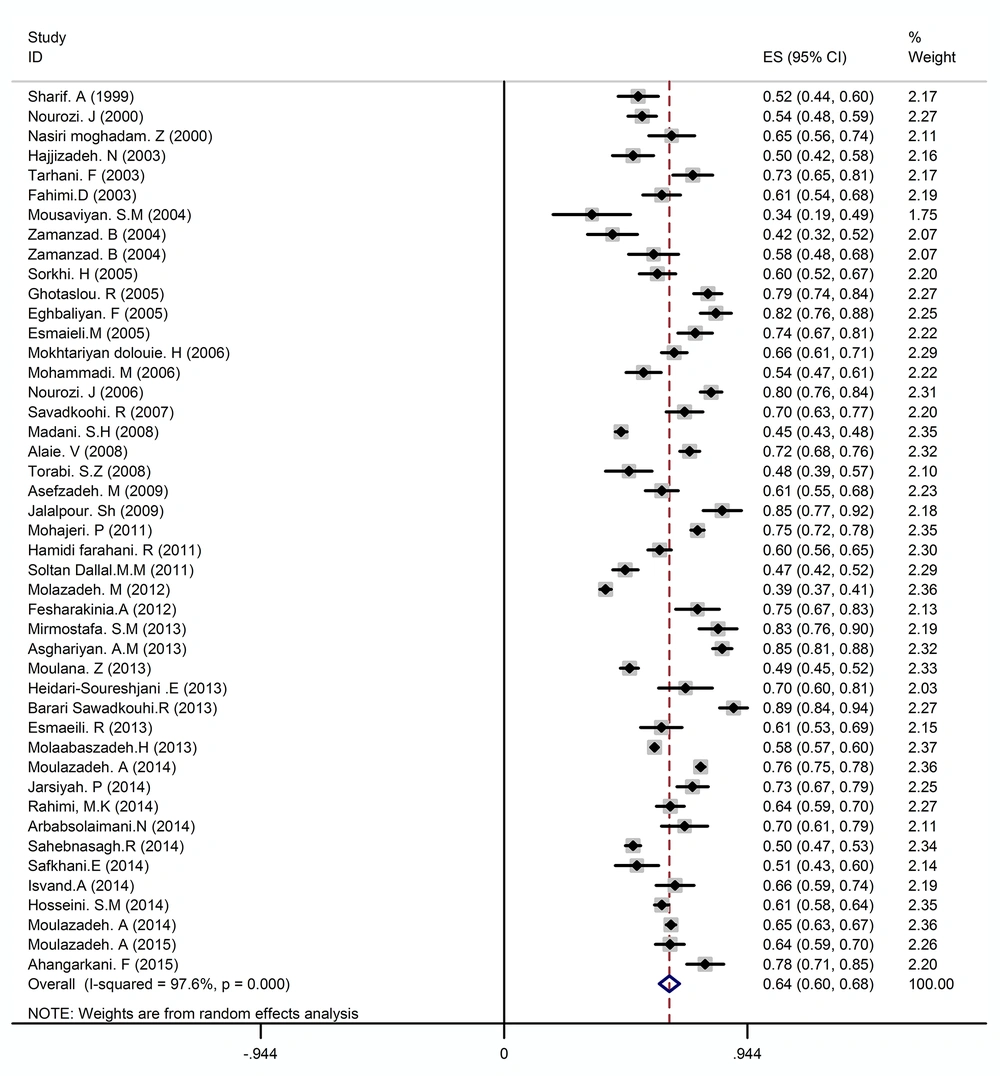
The prevalence of E. coli in urinary tract infections based on random effects model (The midpoint of each line segment shows estimated prevalence rate, the length of line segment indicates a confidence interval of 95% in each study and diamond mark illustrates the prevalence in the whole country for all studies)
Kirby-Bauer disk diffusion test was used to determine the bacterial susceptibility to cotrimoxazole in all studies included in the meta-analysis.
According to the results shown in Table 3, the resistance rate of the most common strains causing urinary tract infection to cotrimoxazole was as follows: E. coli, 62% (with confidence interval: 60 - 65) (Figure 2), Klebsiella, 54% (with confidence interval: 45 - 62), Staphylococcus, 55% (with confidence interval: 47 - 63), and Enterobacter, 52% (with confidence interval: 33 - 70).
| Bacteria Type | The Number of Studies | Sensitive (95% CI) | Semi-Sensitive (95% CI) | Resistant (95% CI) |
|---|---|---|---|---|
| E. coli | 66 | 35 (32 - 389) | 3 (1 - 5) | 62 (60 - 65) |
| Klebsiella | 18 | 46 (37 - 55) | 0 | 54 (45 - 62) |
| Staphylococcus | 9 | 40 (34 - 64) | 5 (3 - 8) | 55 (47 - 63) |
| Enterobacter | 9 | 46 (28 - 65) | 2 (0 - 4) | 52 (33 - 70) |
The Prevalence of Bacterial Resistance to Cotrimoxazole with a 95% Confidence Interval
Since the rate of resistance to cotrimoxazole was highest in E. coli strains in most of the studies included in the meta-analysis, the most common agent causing urinary tract infection was E. coli. Therefore, the ratio of resistance to cotrimoxazole in E. coli strains was studied in different geographical areas of the country. The results showed that the ratio of resistance in the majority of the five regions and cities in the country was high (above 60%) and there were no significant differences between these areas (Tables 4 and 5). It should be noted that the highest resistance rate (88%) was reported in Arak while the lowest rate (22%) was found in Ahvaz.
| City | The Number of Studies | The Prevalence of Resistance% | Confidence Interval (CI%95) |
|---|---|---|---|
| Tehran | 13 | 66 | 57 - 74 |
| Isfahan | 4 | 63 | 65 - 70 |
| Ghazvin | 2 | 54 | 41 - 68 |
| ShahrKord | 3 | 62 | 43 - 80 |
| Arak | 1 | 88 | 79 - 97 |
| Kermanshah | 6 | 61 | 58 - 63 |
| Hamedan | 6 | 54 | 38 - 70 |
| Gharbi Azerbaijan | 1 | 60 | 63 - 58 |
| Zanjan | 2 | 69 | 47 - 92 |
| Tabriz | 6 | 64 | 63 - 65 |
| Ahvaz | 2 | 22 | 1 - 46 |
| Shiraz | 3 | 65 | 58 - 71 |
| Yasouj | 1 | 63 | 54 - 71 |
| Jahrom | 3 | 66 | 49 - 83 |
| Rafsanjan | 1 | 63 | 54 - 70 |
| Mashhad | 2 | 66 | 45 - 86 |
| Semnan | 1 | 42 | 32 - 52 |
| Birjand | 2 | 67 | 61 - 74 |
The Prevalence of Resistance to Cotrimoxazole in E. coli With 95% Confidence Interval in Different Cities of Iran
| Region | The Number of Studies | The Prevalence of Resistance | Confidence Interval (CI%95) | Heterogeneity Index I2 (%) | P-Value |
|---|---|---|---|---|---|
| Central | 22 | 64 | 58 - 71 | 96.3 | < 0.001 |
| North | 9 | 67 | 59 - 75 | 94.7 | < 0.001 |
| West | 19 | 60 | 57 - 63 | 89.8 | < 0.001 |
| South | 10 | 57 | 46 - 67 | 98.3 | < 0.001 |
| East | 6 | 65 | 54 - 76 | 93.2 | < 0.001 |
The Prevalence of Resistance to Cotrimoxazole in E. coli with a 95% Confidence Interval in Different Regions of Iran
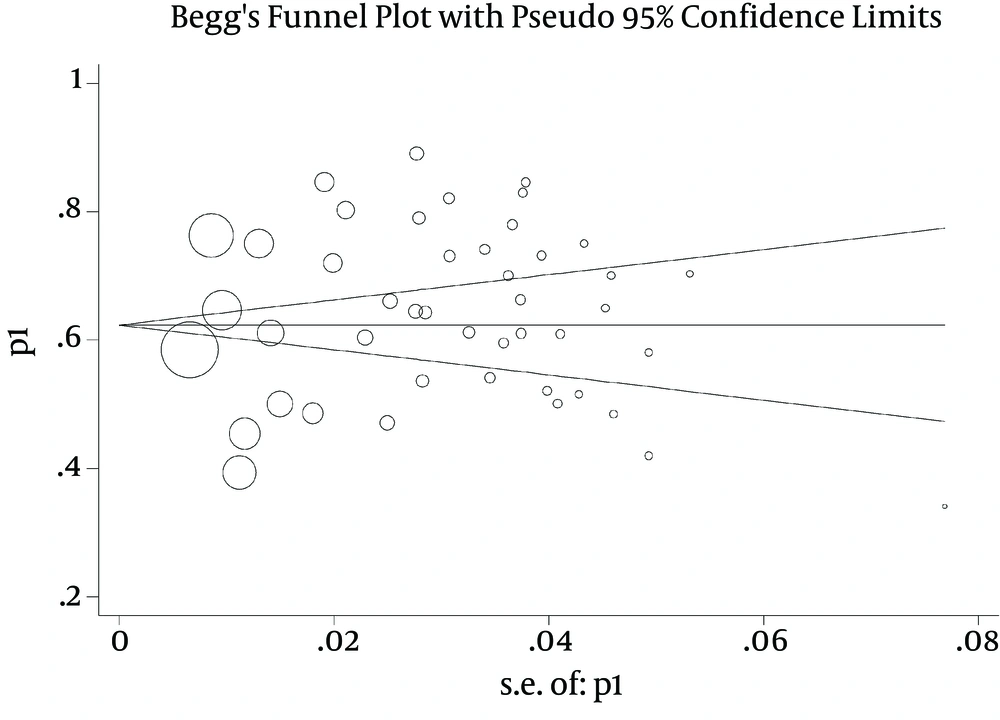
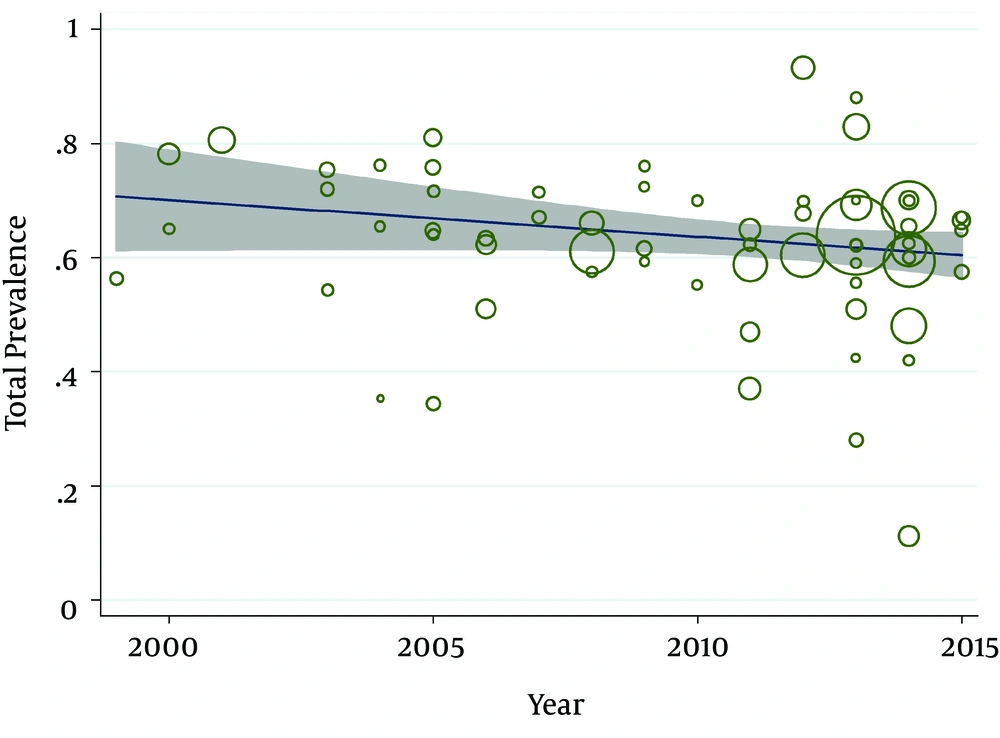
According to the publication bias figure, the effect of bias in these studies was not significant. In fact, most studies were located inside the funnel plot, demonstrating that the results of the most relevant studies performed in Iran were included in the analysis (Figure 3). The interpretation of meta-regression represented that there was no significant relationship between the prevalence of E. coli and year of study (P = 0.15) (Figure 4).
4. Discussion
In the present study, the ratio of urinary tract infection was several times higher in females than in males (78.40% vs. 21.60%, respectively). In other parts of the world, similar results were reported in several studies. For example, Dipiro et al. in New York (78), Boucher et al. in America (79), Linhares et al. in Portugal (80), Garcia-Morua et al. in Mexico (81), Oladeinde et al. and Dibua et al. in Nigeria (82, 83), Chen et al. in Taiwan (84), George et al. in India (85), and Kalsoom et al. in Pakistan (86) have found that the prevalence of urinary tract infection was higher in females. A reason for this issue can be the structure and anatomy of the female genital-urinary tract; the shorter urethra and the proximity of urethral to vaginal and anus in females are the main causes of urinary tract infections in this group (87, 88).
In our society, due to the absence of the culture of correct consumption of antibiotics, most of the patients’ urine cultures were negative because of the previous self-curing. Thus, in many cases, the treatment takes place based on the most prevalent strains of urinary tract infection and their antibiotic susceptibility pattern (63). The results of this research suggest that the bacteria of the Enterobacteriaceae family in most cases are the most prevalent causes of urinary tract infections. Among the members of this family, E. coli is most frequently assigned. These results were consistent with studies conducted in other parts of the world (80, 85, 89-96). In addition, in this study, Klebsiella was the most prevalent one after Escherichia coli, which is in line with other studies (86, 97-100). In most of these studies, in line with the present study, Enterobacteriaceae have been reported as the cause of 90% of the genital-urinary tract infections, with Escherichia coli as the main pathogen, followed by other members of the Enterobacteriaceae family and Gram-positive cocci, especially Staphylococcus. The reason for the higher prevalence of the Enterobacteriaceae family is the presence of these bacteria in the stool and the possibility of urinary tract infection in this way (101). Microorganisms can be compatible with environmental changes in different ways, among which is drug resistance (32). In this study, the most prevalent uropathogens (Escherichia, Klebsiella, Staphylococcus, and Enterobacter) were resistant to cotrimoxazole. Escherichia coli with 62% resistance rate ranked first, followed by other microorganisms. Recent studies show that E. coli isolated from humans is an important pathogen with increased antibiotic resistance to most antimicrobial drugs (49); therefore, the present study focused on the investigation and interpretation of the results of E. coli resistance to cotrimoxazole.
E. coli resistance to cotrimoxazole in most developing countries demonstrated similar results. For example, this resistance has been reported 68.1% in Senegal (46), 58% in Turkey (98), 53% in Lebanon (102), 55% in Brazil (103), and 60% in Mexico (104). E. coli in Bangladesh showed the lowest susceptibility (20 - 27%) to cotrimoxazole (105). In addition, this sensitivity was 33.3% in Ethiopia (100). The results of these studies were consistent with those of the present study and showed the high resistance of E. coli to cotrimoxazole. The susceptibility of E. coli to cotrimoxazole has been reported as 49% in Taiwan (84) and 49.1% in India (85). Comparing the results of the mentioned studies with our findings shows that the prevalence of resistance to cotrimoxazole was higher in our country than in other countries. In all studies cited, empirical treatment with this drug was not satisfactory and it was not recommended to use.
Unlike the present study, the low resistance to cotrimoxazole in E. coli isolates has been reported in some studies, especially in developed countries. For example, the ratio of resistance was 27.1% in Italy (106), 22% in Canada (107), 20.59% in Croatia (108), 21.3% in America (24), 14.5% in Australia (104), 64.1% South Korea (99), and 21% to 36% in several European countries (101). In general, antibiotic resistance in Iran is high compared to some developed countries like the United States (19). The difference could be due to the strains of microorganisms, self-medication by patients, not completing the full course of treatment, inappropriate prescription of antibiotics by physicians, inadequate dose of the drug, drug quality according to the manufacturers, action limited to empirical treatment without regard to culture and susceptibility patterns, poor hygiene, and low social and economic status of life in dirty and crowded environments. According to the high levels of E. coli resistance to cotrimoxazole in this study and similar studies, it seems the phenomenon of drug resistance is increasing in developing countries rapidly. Therefore, accurate strategies by clinical practitioners should be in line with infection control and prevention from the spread of resistance.
The study results conducted in Iran revealed that a low resistance to cotrimoxazole in E. coli strains only was reported in Ahvaz (46, 62) and Semnan (109), which may be due to avoiding the prescription of cotrimoxazole or because of the small number of studies reported in these areas. In all cities in other parts of the country, a high resistance to cotrimoxazole has been reported in E. coli strains. Given these similarities, in the case of the high resistance to cotrimoxazole in different regions of the country, it can be concluded that prescribing patterns and drug use in the country were the same. However, in the past decade, inappropriate use of cotrimoxazole has led to increased resistance to this medication. In most instances, due to the indiscriminate and arbitrary use of antibiotics, many cases of drug resistance can be observed in pathogens leading to treatment failures and many complications, despite the high cost of care. The results of this research are not compatible with the treatment regimen published in 2007 by the world health organization that introduced cotrimoxazole as the first-line drug for the treatment of urinary tract infection (17). According to the results of this study and other studies, it seems that these guidelines need to be revised and even it is suggested developing the first-line treatments specifically for every area based on epidemiological studies. Therefore, according to the results of this study, it can be said that cotrimoxazole is ineffective and its use is not recommended as the first-line antibiotic therapy.
There are several limitations to this study. First, publications from certain provinces were exceedingly high and it might affect the total estimation. Another limitation of this study was a failure to account for the ratio of resistance to cotrimoxazole in strains causing urinary tract infections separately for males and females. Only in a limited number of studies, the resistance was separately calculated for the two genders. Moreover, age is listed among the factors affecting the level of resistance while because of failure to separate the age groups in a number of studies and due to the absence of similar age groups in a number of other studies, we could not calculate the level of resistance in terms of age. In addition, inability to account for the ratio of resistance according to the type of admission was another constraint of the current study since there was no such information in numerous studies. However, our study was the first study that assessed the prevalence of cotrimoxazole resistance in uropathogenic bacteria in Iran and the results of our study are useful in health planning.
4.1. Conclusion
According to the present research, the ratio of urinary tract infections is several times higher in females than in males. E. coli is the most common cause of urinary tract infections, followed by Klebsiella, Staphylococcus, Enterobacter, and other species with slight differences in different geographical areas. All bacteria were resistant to cotrimoxazole. According to the findings of this study, cotrimoxazole is not recommended as the first-line medicine for the treatment of urinary tract infections in Iran.