1. Background
In human, infection with parasites belonging to the genus of Leishmania results in a broad spectrum of diseases, including self-healing cutaneous leishmaniasis, severe mucosal leishmaniasis, and life-treating visceral leishmaniasis. Twelve million people suffer from leishmaniasis worldwide, and each year, two million new cases are diagnosed with the disease, which highlights the need for disease control (1). Among dermotropic species of Leishmania, L. major has a wide distribution throughout Old-World regions (2). The parasite gives rise to skin lesions that eventually heal through the development of cell-mediated immunity (2). In inbreed mice, the deviation of response toward TH1 or TH2 determines the outcome of infection. It has been revealed that TH1 cells produce interferon-γ (IFN-γ) and interleukin-2 (IL-2), and induce cell-mediated immunity and resistance, which lead to the healing phenotype; however, TH2 cells release IL-4 and IL-5, and promote susceptibility to infection (3, 4). The strong immunity provoked by infection with L. major leads to resistance to re-infection, and suggests that vaccination could confer protection against some forms of leishmaniasis. Although no defined vaccine against L. major has been found, there is evidence that leishmanial antigens, such as thiol-specific antioxidant, glycoprotein 63, promastigote surface antigen-2, Leishmania major stress-inducible protein 1, and histone H1, give partial protection in the murine model (5, 6). Therefore, finding new candidate vaccines with the ability to provide full protection is crucial.
In this regard, SOD is an enzyme that scavenges the toxic oxygen radicals, which are responsible for cell damage (7). These SODs comprise a family of metalloenzymes, containing iron (FeSOD), manganese (MnSOD), nickel (NiSOD), or copper, and zinc (Cu/ZnSOD) at the active site. Among them, FeSOD is the main form in parasitic protozoa, including Leishmania species (7). Two types of FeSOD genes, SOD-A and SOD-B, have been cloned from L. major (8). Because of the absence of FeSODs in mammals, FeSODB is an attractive target for a potential therapeutic intervention or vaccine development. The results of previous studies revealed that rSODB1 is immunogenic, since antibody against this protein is present in the sera of 62.5% of patients with visceral and 13.3% of patients with cutaneous Leishmaniasis (9). In addition, formulations of rSODB1 in chitosan nano-particles or with CpG oligodeoxynucleotides or glucopyranosyl lipid adjuvant formulated in a stable emulsion (a synthetic toll-like receptor 4 agonist) adjuvants can increase the IgG2a level in immunized mice (10, 11).
2. Objectives
The aim of the present study was to investigate the efficacy of recombinant SODB1 immunization in the protection of susceptible BALB/c mice against infection with Leishmania major.
3. Methods
3.1. Mice and Parasites
Female BALB/c mice (six to eight weeks), obtained from the Pasture Institute of Iran, were maintained in the animal house during the experiments. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985). The L. major strain MRHO/IR/75/ER parasites were kept in a virulent state through continuous passage in BALB/c mice. The L. major promastigotes were cultured at 26°C in RPMI-1640 (Gibco, USA) medium supplemented with 10% heat inactivated FCS (Gibco, USA), 10mM HEPES, 2mM L-glutamine, and 40 μg/mL gentamycin.
3.2. Recombinant SODB1 Preparation
Preparation of rSODB1 has been described previously (9). Briefly, the coding region of Leishmania major SODB1 was amplified by specific primers, which contained specific sequences for digestion by NdeI (Thermo Fisher Scientific, Lithuania) and XhoI (Thermo Fisher Scientific, Lithuania), as well as the initiation codon. The purified PCR product was cloned directly in pGEM-T easy T vector TA cloning (Invitrogen, USA) and then ligated in the NdeI-XhoI insertion site of expression vector pET22-b (Novagen, USA). The construct was transformed to E. coli BL21 (DE3) and hexa-His-tag fusion protein was expressed by induction with 0.2 mM isopropyl-b-D-thiogalactoside (IPTG). Inclusion bodies were isolated from lysed bacteria and after solubilization in urea (5 M) containing buffer, recombinant protein containing the histidine-tag was affinity purified using IDA-chelating Sepharose (Amersham Pharmacia Biotech AB, Sweden). Purification of recombinant protein was assessed by SDS-PAGE analysis. Purified recombinant protein was gradually dialyzed against guanidine chloride-containing PBS buffer. The concentration of guanidine chloride was 0.5 mM at the end of the experiment.
3.3. Soluble Leishmania Antigen
The Soluble Leishmania Antigen (SLA) was prepared from the stationary phase of L. major promastigotes. The cells were harvested after centrifugation at 4000 × g (Sorvall RC-5 centrifuge, HB-4 swinging bucket rotor) at 4°C for 15 minutes. The pellet was washed with PBS (pH 7.2) and re-suspended in the same buffer to a density of 106 cells/mL. Sonication was used to lyse the cells (four pulses of 1 min using an MSE sonicator with an intensity setting of 15 mm amplitude). The lysate was centrifuged at 17000 × g for 15 minutes at 4°C, and the supernatant was used as SLA. The protein concentrations of the rSODB1 and SLA were measured by the Bradford method.
3.4. Immunization and Challenge with Parasite
Twenty-four mice were categorized to three groups. The first group (immunized group) was immunized with 50 μg of the rSODB1 protein in Complete Freund’s adjuvant (rSODB1+CFA) in a total volume of 100 μL. Control groups received complete Freund’s adjuvant (control group I) or PBS (control group II). Two other subcutaneous booster injections were given in the back of the mice at two-week intervals using incomplete FA instead of complete FA. Three weeks after the second booster injection, the animals were challenged with 1×106L. major meta-cyclic promastigotes (suspended in 50 μL PBS), injected in the right footpad. The progress of infection was monitored for eight weeks by determining the foot-pad swelling, using a metric caliper.
3.5. Cytokine Assay
Exactly before and eight weeks after the challenge, four mice from each group were sacrificed. The splenocytes were cultured using RPMI-1640 medium, supplemented with 10% FCS (Gibco), 2 mM glutamine, 10 mM HEPES, and 40 μg/mL gentamycin. Cells were seeded in triplicates in 96 well plates (Nunc, Denmark) at 2×105 cells/well and stimulated with either rSODB1 (10 μg/mL), or SLA antigen (10μg/mL), or cultured with medium alone. Phytohemagglutinin (PHA), at concentrations of 2 μg/mL, was used in all experiments as a positive control. After 72 hours, supernatants were collected and tested for the IFN-γ, IL-5, and IL-10 production, using the sandwich-based ELISA kits (R&D, Minneapolis, MN, USA), according to the manufacturer’s instructions. The lower detection limits of IFN-γ, IL-5, and IL-10 were 2, 7 and 5 pg/mL, respectively. All experiments were performed in triplicates for four mice.
3.6. Statistical Analysis
The differences in lesions size and cytokine levels were determined by one-way analysis of variance (ANOVA). In case of significant P value, multiple comparisons (Tukey test) were used to compare the means of different treated groups. All statistical analyses were performed using the SPSS software (version 11.5). Differences were considered statistically significant when P < 0.05.
4. Results
4.1. Vaccination with the rSODB1 Induced a Predominant T<sub>H</sub>1 Type Response
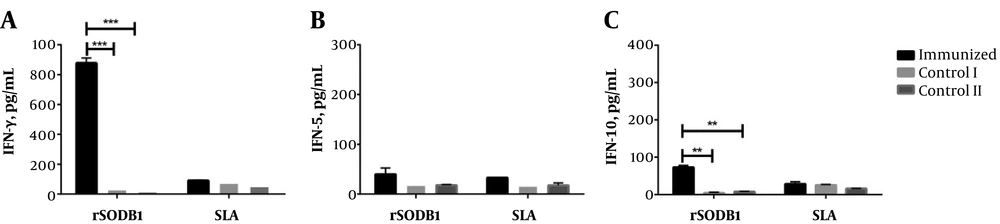
Three weeks after the second booster injection, four mice from each group were sacrificed and splenocytes were removed. In vitro cytokine production was evaluated after stimulation with rSODB1, SLA, and PHA as mitogen. The results showed that IFN-γ concentration was significantly higher in the rSODB1-immunized group when compared to controls, which received either CFA or PBS (878.29 ± 33.40 pg/mL, 32.54 ± 2.46 pg/mL and, 17.66 ± 1.92 pg/mL respectively, P = 0.0001; Figure 1A and Table 1). On the other hand, IL-5 levels did not show any significant difference between vaccinated and control groups (39.86 ± 12.48 pg/mL, 18.68 ± 5.53 pg/mL and, 17.75 ± 1.62 pg/mL, respectively; P = 0.23; Figure 1B and Table 1). Additionally, after in vitro re-stimulation with rSODB1, significantly higher levels of IL-10 were detected in the supernatant of splenocytes isolated from rSODB1+CFA immunized animals when compared to those treated with CFA or PBS (Figure 1C and Table 1).
Cytokine production in mice before challenge with L. major promastigotes. The splenocytes were stimulated in vitro with rSODB1 (10 µg/mL) and SLA (10 µg/mL), after 48 hours, supernatants were harvested and cytokine levels of IFN-γ (A), IL-5 (B) and IL-10 (C) were measured by ELISA. Each bar represents mean values ± standard deviation in pg/mL. P ≤ 0.05, P ≤ 0.01 and P ≤ 0.001 is shown by *, ** and ***, respectively. The scale bar was adjusted with Figure 3.
| rSODB1+CFA | CFA | PBS | P1a | P2b | ||||
|---|---|---|---|---|---|---|---|---|
| rSODB1 | SLA | rSODB1 | SLA | rSODB1 | SLA | |||
| Pre-challenge | ||||||||
| IFN-γ, pg/mL | 878.3 ± 33.4 | 91.0 ± 3.7 | 32.5 ± 2.5 | 76.4 ± 10.2 | 17.7 ± 1.9 | 53.53 ± 12.6 | < 0.0001 | 0.15 |
| IL-5, pg/mL | 39.9 ± 12.4 | 33.1 ± 0.4 | 18.7 ± 5.5 | 17.0 ± 0.8 | 17.8 ± 1.6 | 17.5 ± 5.0 | 0.23 | 0.05 |
| IL-10, pg/mL | 72.8 ± 4.9 | 28.3 ± 5.9 | 4.1 ± 2.1 | 25.1 ± 2.2 | 7.7 ± 1.2 | 15.7 ± 0.4 | 0.001 | 0.18 |
| IFN-γ/IL-5 | 24.7 ± 8.5 | 2.8 ± 0.2 | 1.9 ± 0.7 | 4.5 ± 0.8 | 1.0 ± 0.02 | 3.6 ± 1.7 | 0.007 | 0.60 |
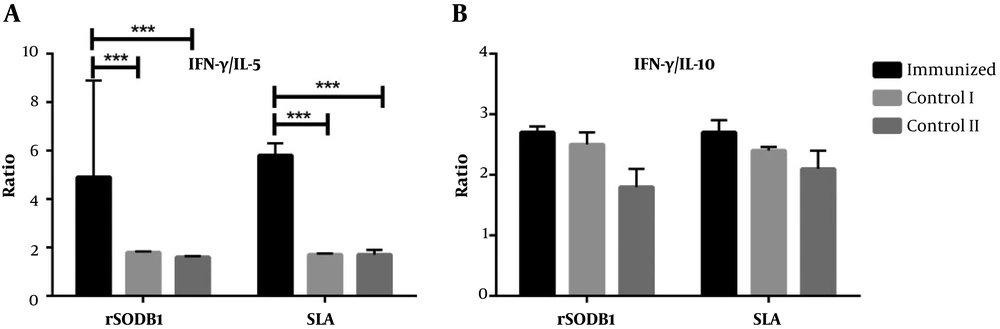
| IFN-γ/IL-10 | 12.2 ± 1.3 | 3.4 ± 0.8 | 10.4 ± 4.8 | 3.0 ± 0.1 | 2.3 ± 0.1 | 3.4 ± 0.9 | 0.17 | 0.9 |
| Post-challenge | ||||||||
| IFN-γ, pg/mL | 560.7 ± 34.0 | 810.7 ± 62.3 | 333.7 ± 2.7 | 423.6 ± 8.0 | 299.0 ± 24.4 | 430.0 ± 22.4 | 0.009 | 0.009 |
| IL-5, pg/mL | 115.8 ± 2.1 | 145.8 ± 2.9 | 190.1 ± 3.3 | 254.9 ± 12.8 | 182.4 ± 9.6 | 252.0 ± 18.7 | 0.005 | 0.016 |
| IL-10, pg/mL | 211.7 ± 2.6 | 298.3 ± 5.5 | 135.2 ± 11.4 | 178.4 ± 8.0 | 170.1 ± 10.5 | 212.1 ± 16.9 | 0.02 | 0.01 |
| IFN-γ/IL-5 | 4.9 ± 0.4 | 5.8 ± 0.5 | 1.8 ± 0.04 | 1.7 ± 0.05 | 1.6 ± 0.05 | 1.7 ± 0.2 | 0.003 | 0.006 |
| IFN-γ/IL-10 | 2.7 ± 0.1 | 2.7 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.06 | 1.8 ± 0.3 | 2.1 ± 0.3 | 0.098 | 0.18 |
Cytokines Levels (Mean ± SEM) After Stimulation of Splenocytes with rSODB1 and SLA in Pre-Challenged and Post-Challenged Mice Immunized with rSODB1+CFA Compared with CFA- or PBS-Treated Control Groups
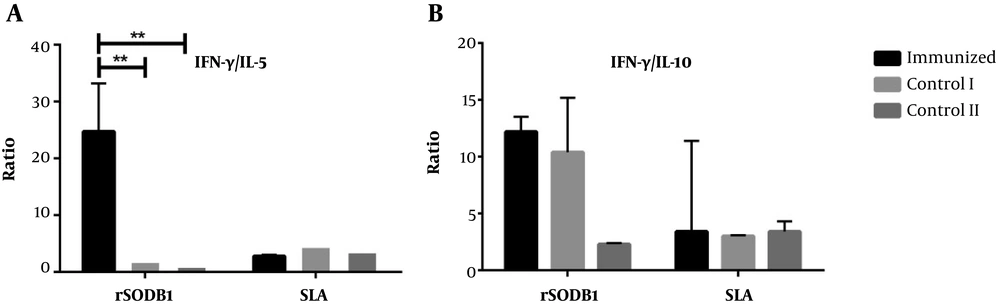
Of interest, the ratio of IFN-γ/IL-5 in rSODB1-vaccinated group was also higher compared to the control groups, though this difference did not reach the significant levels (24.7 ± 8.5 pg/mL, 1.95 ± 0.7 pg/mL and 1.0 ± 0.02 pg/mL, respectively; P = 0.07; Figure 2A). However, after re-stimulation with rSODB1, the ratio of IFN-γ/IL-10 was significantly different between study groups (Figure 2A and Table 1).
Interestingly, after in vitro re-stimulation with SLA, the production of IFN-γ in rSODB1-immunized groups were not significantly different with CFA or PBS, while the levels of IL-5 were significantly higher than CFA- or PBS-treated controls. This is despite the fact that the production of IL-10 and the ratio of IFN-γ/IL-10 after re-stimulation with SLA were not statistically different among the vaccinated group and CFA- or PBS-treated groups.
4.2. Vaccination with rSODB1 Prevent an Excessive T<sub>H</sub>2 Response Formation After Challenge with L. major
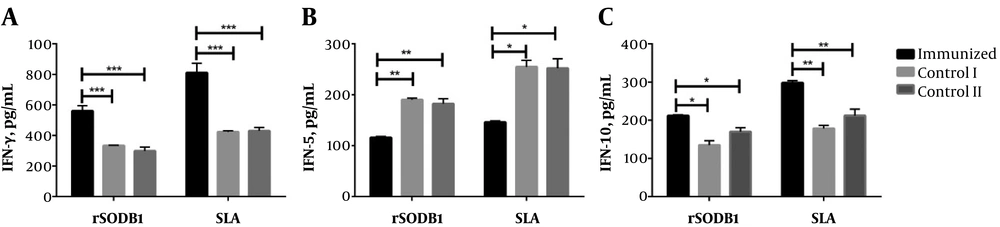
Eight weeks after infection, splenocytes were re-stimulated in vitro with rSODB1 or SLA and the culture supernatants were used to measure the cytokine levels. As shown in Figure 3A, the level of IFN-γ, which was produced in response to rSODB1 by splenocytes from immunized mice, was significantly higher than controls treated with CFA or PBS alone (560.6 ± 34.00 pg/mL, 333.74 ± 2.72 pg/mL, and 299.02 ± 24.4 pg/mL, respectively; P = 0.009). In the same manner, re-stimulation of splenocytes with SLA, produced significantly higher IFN-γ levels compared to mice treated with CFA or PBS. Additionally, the level of IL-5 production after re-stimulation of splenocytes by either rSODB1 or the SLA was significantly lower in rSODB1+CFA-vaccinated mice compared to CFA or PBS treated groups (for rSODB1: P = 005; for SLA: P = 0.016; Figure 3B). However, IL-10 production in immunized mice showed a significant increase in compared to mice treated with CFA or PBS (P = 0.01, Figure 3C).
In vitro cytokine production levels by splenocytes isolated from mice that were challenged with L.major promastigotes. Eight weeks after the challenge, four mice from each group were sacrificed, and the splenocytes were stimulated with rSODB1 (10 µg/mL) or SLA (10 µg/mL). After 48 hours, supernatants were collected and the levels of cytokine including IFN-γ (A), IL-5 (B) and IL-10 (C) were measured by ELISA. Each bar represents mean values ± standard deviation in pg/mL. P ≤ 0.05, P ≤ 0.01 and P ≤ 0.001 is shown by *, ** and ***, respectively.
The IFN-γ/IL-5 ratio was significantly higher after re-stimulation with both rSODB1 and SLA in vaccinated mice compared to control groups (for rSODB1: P = 0.003; for SLA: P = 0.006; Figure 4A). Due to the fact that both IFN-γ and IL-10 were up-regulated after re-stimulation with rSODB1 or SLA in challenged mice, the ratio of IFN-γ/IL-10 did not show any significant differences between vaccinated and CFA- or PBS-treated groups (Figure 4A).
4.3. Effect of Vaccination with rSODB1 on Footpad Lesions in Mice Challenged by L. major
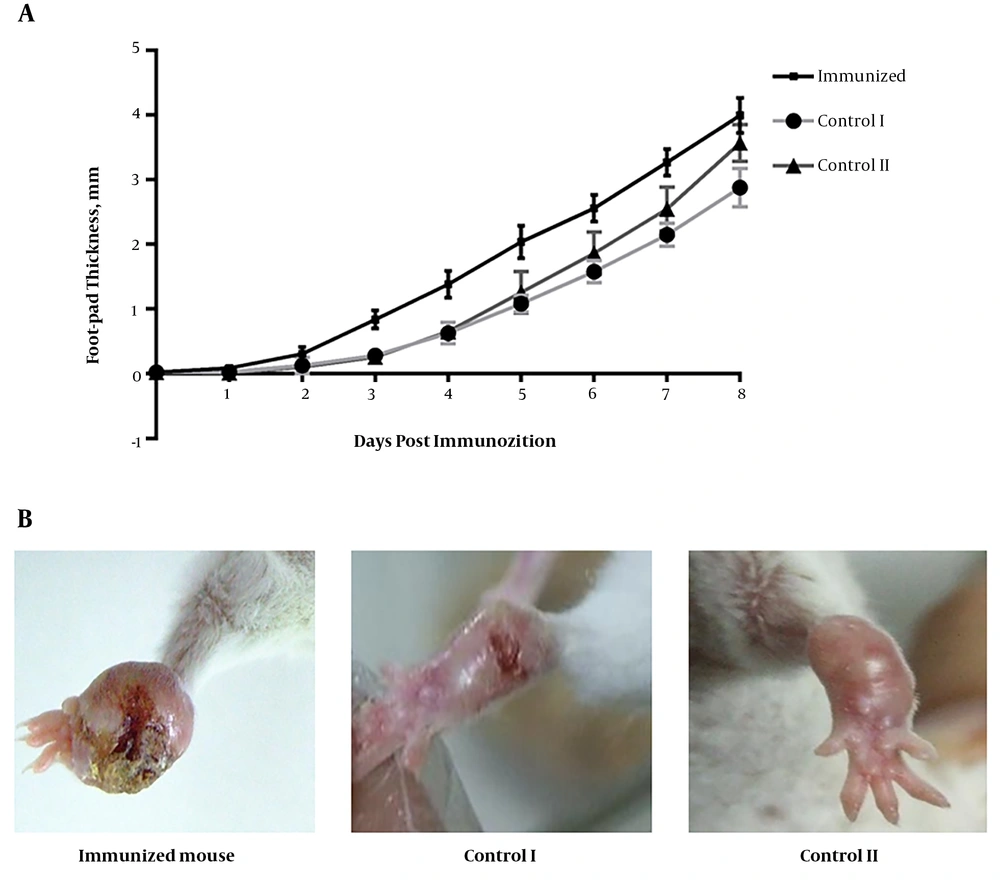
To evaluate whether the activated TH1 response was able to alter the course of the disease, delayed-type hypersensitivity responses in the foot-pad of infected mice were monitored. TH1 mice were challenged three weeks after the last booster injection with 1×106L. major stationary phase promastigotes and the course of the infection was followed for eight weeks. Immunization with the rSODB1 induced the onset of swelling one week earlier, compared to CFA- or PBS-treated mice. Thereafter, lesion size progressed more rapidly in the mice vaccinated with rSODB1 plus FA (P = 0.03). The lesion size at the end of eight weeks of challenge was significantly larger in the immunized group compared with those that received the adjuvant (P = 0.0001, Figure 5). However, no differences were observed between the vaccinated group and PBS-treated controls. In fact the mice in the vaccinated group were ultimately not able to control the infection and complete necrosis of the infected footpad were also observed at the end of the eighth week post challenge.
The effect of immunization with rSODB1on footpad swelling of L. major infected mice. A, foot-pad swelling was measured weekly after L. major challenge in vaccinated and control mice. Results are shown as mean ± S.E; B, the observed lesions in the foot-pad of the mice 8 week after challenge. Images are representative of results obtained from 4 independent experiments.
5. Discussion
The findings of the present study showed that immunization of BALB/c mice with rSODB1+CFA induced an immune response characterized by higher levels of IFN-γ and IL-10, in comparison to controls that received CFA or PBS. Of interest, after challenging with virulent parasite, re-stimulation of splenocytes with rSODB1 or SLA still induced higher levels of both IFN-γ and IL-10 in rSODB1+CFA vaccinated mice compared with CFA- or PBS-treated mice, though the levels of IL-5 was significantly lower in the supernatant of splenocytes derived from vaccinated groups compared to controls. In line with these findings, the ratio of IFN-γ/IL-5 (as a representative of TH1/TH2 ratio) was also significantly higher in rSODB1+CFA immunized group compared to those of CFA- or PBS-treated groups.
Previously, the current researchers demonstrated that rSODB1 is an immunogenic protein since it is recognized by 62.5% of sera from patients infected with the visceral form of leishmaniasis (9). Additionally, immunization of BALB/c mice with rSODB1 plus chitosan nanoparticles or synthetic toll-like receptor 4 agonist leads to the production of anti-rSODB1 TH1-associated IgG2a antibodies (10, 11). These findings proposed that SODB1 could be considered as a candidate vaccine in leishmaniasis. Interestingly, the results of the present study showed that immunization with rSODB1+CFA could induce a TH1-biased immune response after in vitro re-stimulation with rSODB1 or SLA (Table 1). However, while the TH1-dominant immune response remained stable after challenge with virulent parasite, the immunized animals were not protected against Leishmania infection compared to those that received CFA or PBS. In fact, the current results showed that after challenge with parasites, rSODB1+CFA-immunized mice had earlier onset of swelling, which more rapidly progressed in size compared to the CFA-treated group.
The widespread negative role of IL-10 in Leishmania vaccine failure is indeed well-established (12). It has been shown that IL-10 suppresses the functions of effector cells that are necessary for the induction of cell-mediated immunity; those necessary to harness many intracellular pathogens, such as Leishmania spp. (13). Additionally, some researchers have reported that the increased levels of IL-10 were closely associated with severity of visceral leishmaniasis in humans (14, 15). The IL-10 production also correlates with lesion progression in patients with cutaneous leishmaniasis (16, 17). Interestingly, Roberts et al. showed that higher levels of both IL-4 and IL-10 relative to IFN-γ was associated with exacerbation of L. major infection (18). In addition, IL-10 is also responsible for disease exacerbation in resistant mice (19). In this respect, Nashed et al. showed that in DBA/2 mice (resistant phenotype), which were immunized by using a mixture of SLA and incomplete Freund’s adjuvant, the up-regulation of IL-10 was specifically associated with the exacerbation of infection (19). Therefore, it can be concluded that some Leishmania antigens may suppress the TH1 immune response through up-regulation of the IL-10 level. The results of the current study showed that the biased-TH1 response in both pre-challenged and post-challenged BALB/c mice after immunization with rSODB1+CFA compared to CFA administration is accompanied with higher levels of IL-10 production (Table 1). Accordingly, more rapidly developed footpad lesions in the presence of a TH1-dominant immune response can be explained based on the simultaneous presence of higher levels of IL-10 in rSODB1+CFA-immunized mice compared to CFA-administered mice. Therefore, consistent with other studies (18), the current results also suggest that IL-10 is a major determinant of L. major disease progression in rSODB1+CFA immunized mice.
In conclusion, the current results suggest that the recombinant SODB1 did not induce protection in susceptible BALB/c mice when administrated with complete Freund adjuvant. In addition, the results of this study showed that the induction of a polarized TH1 response or prevention of an aberrant TH2 response may not be sufficient for resistance to Leishmania infection and support the hypothesis that high efficacy vaccines targeting Leishmania should be able to simultaneously shifts the balance from TH2 to TH1 and inhibit the production of IL-10.