1. Background
Helicobacter pylori, spiral Gram negative and microaerophilic bacteria, are present in the gastric mucosa of most of the world’s population (1). They were introduced as a class I carcinogenic agent in 1994 by the world health organization (WHO) (1). These bacteria induce chronic inflammation leading to chronic gastritis, gastric ulcer, duodenal ulcer, and ultimately cancer (2). They are rapidly becoming resistant to most antibiotics used in therapeutic regimens.
The extent of antibiotic resistance patterns varies in different geographical regions, which is a direct reflection of the antibiotic regimens used in each region (3). The most common regimen includes a bismuth salt along with 2 of the following antibiotics: metronidazole, amoxicillin, clarithromycin or tetracycline. However, antibiotic resistance is becoming very prevalent in developing countries. Antibiotic resistance is assumed to be the main reason of eradication failure in H. pylori infected patients (4). Since metronidazole is the most common antibiotic used, especially in developing countries, metronidazole resistance reduces treatment adequacy by 50% in these areas (5). The high level resistance to this drug has led to elimination of many first line antibiotics (6). Different alternative regimens are now proposed for empirical first line therapy against H. pylori infection in high clarithromycin and metronidazole resistance areas. Nitazoxanide and doxycycline are 2 antibiotics that their use together with quinolones was suggested as a successful alternative therapy (7). Nitazoxanide, a benzamide thiazolide derivative, has been shown to be effective against many bacteria as well as helminthes and protozoa (8-10). It is believed to act through interference with pyruvate metabolism, which seems to be required for anaerobic cell energy metabolism (8). Doxycycline, which is a synthetic tetracycline derivative is absorbed more easily with food and has a much simpler dosing schedule (11). Adverse side-effects of doxycycline is lower than the numerous side-effects of tetracycline, such as tooth discoloration, gastrointestinal symptoms, candidiasis, and photosensitivity (11-14). Due to the worldwide high prevalence of metronidazole resistance H. pylori strains, this study sought to determine the in vitro effectiveness of nitazoxanide and doxycycline against metronidazole resistant isolates at different concentrations.
2. Objectives
This study aimed at investigating antibiotic resistance patterns of metronidazole resistant H. pylori strains to doxycycline and nitazoxanide.
3. Methods
3.1. Patients, Sample Collection and Culture
This cross sectional study was undertaken at 3 general hospitals of Tehran from November 2014 to July 2015. Helicobacter pylori strains were isolated from 122 biopsy specimens obtained from patients with gastrointestinal complaints during endoscopy. Three biopsies were obtained from antrum of stomach by a gastroenterologist, which were used for pathological analysis and examination of urease activity and culture. One of the samples was immediately transferred to the laboratory in thioglycolate medium (MERCK, Germany) for culture. The sample was subsequently processed for bacterial culture in Brucella agar medium supplemented with 10% horse blood, 10% fetal bovine serum, amphotericin B (10 mg, Sigma-Aldrich, USA), and Campylobacter selective supplement consisting of vancomycin 2.0 mg, polymyxin 0.05 mg, and trimethoprim 1.0 mg (Merck, Homburg, Germany). The plates were incubated at microaerophilic atmosphere (%8 N2, %5 CO2, %8 - 10% O2) in a Gas Pak jar for 3 to 5 days. Exclusion criteria included treatment with proton pump inhibitor and use of antibiotics during the two last weeks prior to sample collection.
3.2. Isolation and Characterization of Helicobacter pylori Strains
Grown colonies of bacteria were examined macroscopically and biochemically. Small shiny colonies that showed positive urease, catalase, and oxidase tests were collected and preserved at -20°C for molecular identification using species specific primers. Freshly grown colonies of the bacterial isolates were subjected to QIAamp tissue DNA extraction kit, according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Polymerase Chain Reaction was performed by primer targeting glmM (ureC), which amplified a 296-bp fragment based on PCR conditions, as described by Shahabimehr et al. (14).
3.3. Determination of Minimum Inhibitory Concentrations
To measure the lowest inhibitory concentrations of the antibiotics for H. pylori strains, minimal inhibitory concentrations were determined by the agar dilution method, according to the CLSI guidelines (15). Accordingly, a suspension with a turbidity equivalent to that of a McFarland No.2 standard (approximately 6 × 108 bacteria/mL) was prepared from a 48-hour culture on agar plates. Susceptibility testing was performed on Mueller Hinton agar medium containing horse blood (10%) and appropriate dilution of metronidazole (0.5 to 64 µg/mL). Following preparation of microbial suspension, inoculation took place in plates containing different concentrations of metronidazole by spotting 10 µL of the bacterial suspension. Within a short space of time after the absorption of the samples, plates were incubated under microaerophilic conditions for 2 days at 37°C. Minimum inhibitory concentration was determined as the lowest concentration of antimicrobial agent inhibiting the total growth of bacteria. Resistance was considered when the metronidazole MIC was greater than 8 µg/mL (Eucast, version 2.0). After identification of metronidazole resistant isolates, the resistant strains were tested for determination of the MIC values of doxycycline and nitazoxanide. The same protocol was used for determination of MIC values of nitazoxanide and doxycycline, except the bacterial turbidity that was set at McFarland No.3 standard (approximately 6 × 109 bacteria/mL) for nitazoxanide. Doxycycline concentrations ranged from 0.12 to 8 µg/mL and nitazoxanide concentrations ranged from 0.03 to 32.0 µg/mL. Minimum inhibitory concentration values at which 50% and 90% of the tested strains were inhibited, were reported as MIC50 and MIC90, respectively. Dimethyl sulfoxide was used as the solvent for providing defined concentrations of doxycycline and nitazoxanide. Metronidazole, doxycycline, and nitazoxanide were obtained from Sigma Company (USA). Since there were no acceptable breakpoints for interpretation of nitazoxanide and doxycycline resistance for H. pylori strains, MIC values were reported for these two antibiotics. Helicobacter pylori strain RIGLD OC218 was used as a control strain in all experiments.
3.4. Statistical Analysis
Results of the demographic information and values of MICs of antibiotics were analyzed using the SPSS 18 software (SPSS, Chicago, IL, USA). Also, chi-square test and Fisher’s exact test were used to analyze the data. A p value of ≤0.05 was considered statistically significant for differences.
4. Results
4.1. Helicobacter pylori Infection
Out of 122 gastric biopsy specimens, 55 H. pylori isolates were collected (45%). Identity of all the isolates was confirmed as H. pylori by both biochemical and molecular methods. The mean age of infected patients was 49 years (males: 49% and females: 51%). No significant difference was detected in the infection rate between males and females (54.5%, 30/55 and 45.4%, 25/55, respectively). Pathological findings showed occurrence of chronic gastritis (58%), severe active gastritis (30%), and intestinal metaplasia (12%) in these patients.
4.2. Quantitative Method
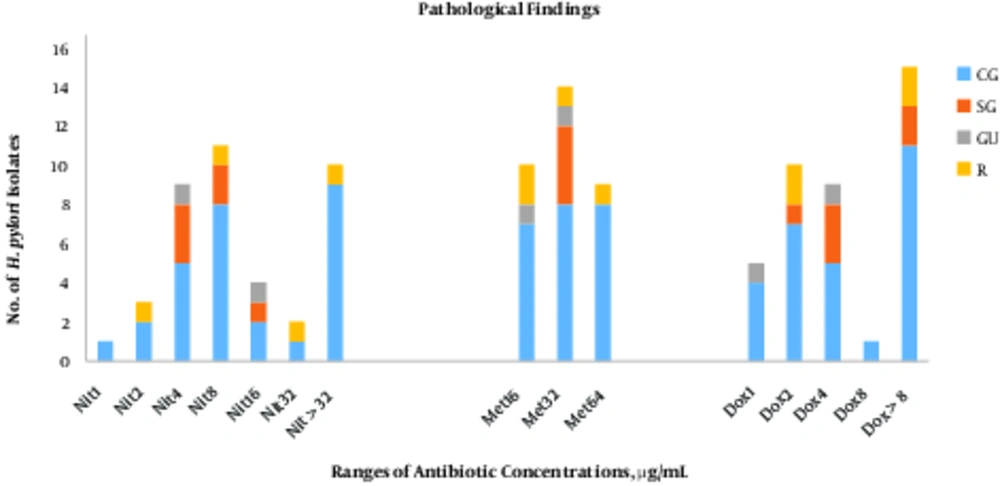
There was no significant correlation between MIC values of 3 antibiotics and pathological findings.
4.3. Antibiotic Susceptibility Testing
Results of antibiotic susceptibility testing showed a frequency of 60% (33/55) for metronidazole resistant H.pylori strains (MIC > 8µg/mL). The MIC values of metronidazole for the H. pylori strains are shown in Table 1.
| Metronidazole, µg/mL | Nitazoxanide, µg/mL | Doxycycline, µg/mL | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | ≥32 | 1 | 2 | 4 | ≥8 | ||
| 16 | 0 | 1 | 1 | 3 | 1 | 4 | 1 | 2 | 2 | 5 | 10 (30.3) |
| 32 | 0 | 2 | 3 | 3 | 1 | 5 | 3 | 0 | 4 | 7 | 14 (42.5) |
| 64 | 1 | 0 | 3 | 2 | 1 | 2 | 1 | 4 | 1 | 3 | 9 (27.2) |
| Total | 1 (3) | 3 (9) | 7 (21.3) | 8 (24.4) | 3 (9) | 11 (33.3) | 5 (12.5) | 10 (25) | 9 (22.5) | 16 (40) | 33 (100) |
aValues are expressed as No. (%).
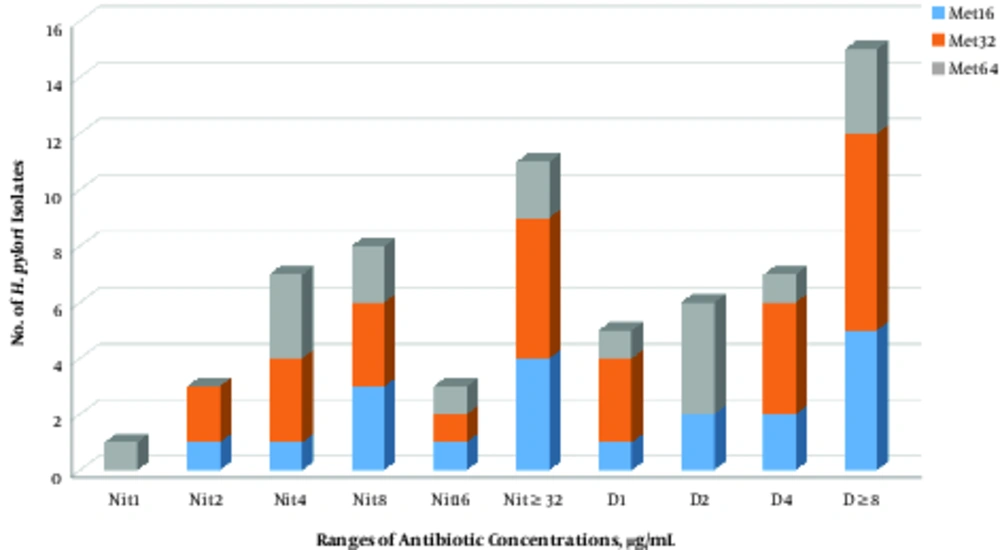
The MIC50 and MIC90 values for metronidazole were 32 and 64 µg/mL, respectively. In the case of doxycycline, its MIC values ranged between 1 and ≥8 µg/mL. The MIC50 and MIC90 values for doxycycline were measured as 4 and ≥8 µg/mL, respectively (Table 1). The MIC values for nitazoxanide in the metronidazole resistant H. pylori were in ranges of 1 to ≥32 µg/mL. The MIC50 and MIC90 values for nitazoxanide were determined as 8 and ≥32 µg/mL, respectively. Diversity of MIC values of doxycycline and nitazoxanide for all of the metronidazole resistant strains is shown in Figure 1. In general, higher activity of nitazoxanide and doxycycline was detected against metronidazole resistance H. pylori strains. Although H. pylori strains with an MIC value of ≥32 µg/mL for metronidazole were mainly inhibited with lower concentrations of nitazoxanide and doxycycline, this correlation was not found for the strains with lower MIC values (8 to 16 µg/mL). Accordingly, some of the metronidazole resistant strains with MIC values of ≥8 µg/mL were not inhibited by highest examined concentrations of these 2 antibiotics.
5. Discussion
Helicobacter pylori is an important human pathogen around the world. The prevalence of H. pylori infection in Iran, like other developing countries, is higher than those in the developed world (16-23). The prevalence of H.pylori infection in different areas of Iran is variable, ranging from 30.6% to 93.0% (19-21). In this study, the estimated infection rate was 45%, which is similar to that of other researchers reported from Tehran (24-26). Antibiotic resistance is the main reason for treatment failure and persistent infection of H. pylori in developing countries. The increasing resistance to antibiotics is commonly due to a lack of proper administration, inappropriate dosing, arbitrary use of drugs, and genetic mutations in H. pylori strains (27, 28). Metronidazole is a common antibiotic used for H. pylori treatment in Iran, and resistant strains to this antibiotic are becoming a major problem for therapeutic regiments (27). Resistance to metronidazole was raised among H. pylori strains in Iran from 73.4% in 2009 to 88.2% in 2011 (27). In the present study found high rates of metronidazole resistant strains of H. pylori (60%) in the studied hospitals. This resistance rate seems to be associated with historical administration of metronidazole for various parasitic and oral cavity infections, since most of the strains were isolated from patients over 40 years old. While no statistically significant change in resistance rate was detected for metronidazole in this study, a higher amount of MIC values (MIC50 ≥ 64 µg/mL vs 32 µg/mL) was found compared with previous studies from Iran (28).
Tetracyclines are other anti-H. pylori antibiotics, which are used as a component of the quadruple therapy regimens. The extent of resistance towards this drug is also variable in different areas. This discrepancy may in-part be due to different sample sizes and the specific antibiogram method used (18, 19). There are many reports indicating an increasing trend of resistance towards this antibiotic; however, resistance rate to this antibiotic is lower than those described for metronidazole (29-37). Comparison of MIC50 for doxycycline and metronidazole (4 vs 32 µg/mL) proposed 8 times higher activity of doxycycline against metronidazole resistant H. pylori strains. However, nitazoxanide showed a four-fold greater anti-bacterial property compared with metronidazole, based on its MIC50 value. It could therefore be suggested that these 2 drugs were superior in their ability to inhibit growth of H. pylori in comparison to metronidazole. There are a few studies on the activity of doxycycline and nitazoxanide against metronidazole resistant strains. In a study by Cammarota et al. in Italy, superior effects of doxycycline-based quadruple regimen for eradication of metronidazole resistant H. pylori infection, with MIC range of 0.056 to 1 µg/mL and MIC50 and MIC90 of 0.125 and 0.5 µg/mL, was presented (38). The MIC range of 0.25 to 8.0 µg/mL for doxycycline and MIC50 and MIC90 of 0.5 and 2 was also reported for clinical isolates of H. pylori in Canada (39). Results of the current study showed higher MIC ranges for this antibiotic (MIC range of 1 to ≥8.0 µg/mL and MIC50 and MIC90 of 4 and ≥8), which suggests greater consideration for its administration to prevent emergence of more resistant strains in Iran. In case of nitazoxanide, Megraud F. et al reported MIC range of 0.25 to 8 µg/mL, with MIC50 and MIC90 of 1 and 4, among metronidazole resistant and susceptible strains (0.25- >32, MIC50 and MIC90 2 and >32 µg/mL) (40). In a study from Australia, MIC range of nitazoxanide varied between 0.06 and 4 µg/mL (41). Likewise, these MIC ranges were lower than those detected in Iran. However, they observed no significant change in MIC levels after nitazoxanide administration compared with metronidazole, and its higher activity against H. pylori strains suggests its preference for clinical usage.
Lack of association between MIC values of metronidazole and those from nitazoxanide or doxycycline could be explained by diversity of resistance mechanisms to these drugs (35). Although the current results provide some evidence about preferred activity of nitazoxanide and doxycycline, further studies are needed to investigate their usage against metronidazole resistance H. pylori strains.