1. Background
Tuberculosis (TB) remains a major cause of morbidity and mortality and a public health burden in the developing countries, which is estimated to be responsible for nearly 1.4 million deaths with 8.7 million annual new cases (1). The causative agent of TB is Mycobacterium tuberculosis (MTB), which is a slow-growing facultative pathogen (2). According to the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains of MTB, as well as the growing spread of human-immunodeficiency virus (HIV) among patients with TB, the situation of TB is worsening worldwide (3, 4). Early diagnosis and consequent interventions are needed to effectively control TB and provide prognostic information (5, 6). T-cell-based assays such as highly sensitive and specific interferon-gamma release assay (IGRA) (7) are commonly used as gold standard tests for the detection of TB (8) and could be a potential diagnostic marker for other inflammatory diseases (9). However, this expensive and complicated assay is not capable of distinguishing active and latent TB infections (10). Therefore, there is an urgent need for more convenient and inexpensive TB biomarkers (11). Cytokines, as key regulators of immune responses, have a major role in determining the fate of host-pathogen interaction (12). The alterations of these molecules could reflect the status of disease (13). Thus cytokines could be introduced as potential diagnostic and prognostic TB biomarkers (14, 15). Numerous cytokines have been introduced by Chegou et al. as potential diagnostic markers for distinguishing active TB from its latent form (16).
Interleukin-6 (IL-6) is a pro-inflammatory cytokine, which is secreted by T cells and macrophages (17). It is probably involved in the pathogenesis of TB by stimulating the secretion of IFN-γ, which plays a crucial role in the activation of MTB-infected macrophages (18). Interleukin-17 (IL-17) is also a pro-inflammatory cytokine, which is known for its crucial role in immune responses against extracellular pathogens (19). Despite the overexpression in the patients with active TB, IL-17 may play a role in protective immunity against MTB infection (20). Transforming growth factor-beta (TGF-β) is a multifunctional cytokine, which is produced by T cells, B cells, and myeloid cells with different functions (21). TGF-β may be involved in the suppression of T cell responses, macrophage deactivation, and tissue injury initiation and deterioration (22).
2. Objectives
In the present study, we examined the plasma expression of IL-17, IL-6, and TGF-β among newly diagnosed (ND) and under treatment (UT) patients with TB in comparison to the normal subjects and analyzed the diagnostic utility of each cytokine to introduce a novel TB biomarker.
3. Methods
3.1. Sample Collection
We recruited 105 smear positive, including 78 newly diagnosed (ND) and 27 under treatment (UT) patients with pulmonary TB from public health centers and educational hospitals of Golestan University of Medical Sciences, Gorgan, Iran. Patients with pregnancy and chronic inflammatory disorders, including diabetes, autoimmune diseases, cancer, allergic diseases, immunocompromised conditions, coinfections with any other infectious diseases such as viral hepatitis, and heart failure were not included in this study. Moreover, the patients receiving the treatment either self-medicated or prescribed by the physician were excluded from the ND group. Accordingly, a total of 111 age- and sex-matched healthy subjects were enrolled. The healthy controls were also evaluated for all mentioned inflammatory conditions and the individuals with a history of pulmonary disorders in their family were not included. Written informed consent was taken and signed by all participants in compliance with the Declaration of Helsinki. Whole blood samples were taken from all subjects, plasma was separated and stored at -80°C until the measurement of cytokines.
3.2. ELISA Cytokine Assay
The commercially available ELISA kits were used to determine the plasma level of IL-17, IL-6 (Biolegend, CA, USA), and TGF-β (eBioscience, San Diego, USA) in patients with TB and healthy subjects, according to the manufacturer’s instructions. The optical densities of all samples were obtained at the wavelength of 450 nm using Biotek ELISA reader ELX800 (Biotek, VT, USA), as previously described (23, 24). All samples were measured in duplicates and the results were reported as pg/mL.
3.3. Statistical Analysis
In order to analyze data statistically and prepare graphs, SPSS V. 22.0 and Graphpad Prism V. 5.04 software were used. Shapiro-Wilk test was used to address the normal distribution of variables in all groups. All data were demonstrated as mean ± SE (standard Error). Significant differences were also assessed using independent samples Mann-Whitney U test for two-group comparisons. The non-parametric Kruskal-Wallis with Dunn-Bonferroni post hoc test was also used to compare the means of multiple samples. P values less than 0.05 were considered statistically significant.
4. Results
4.1. ELISA Expression of IL-6, IL-17 and TGF-β
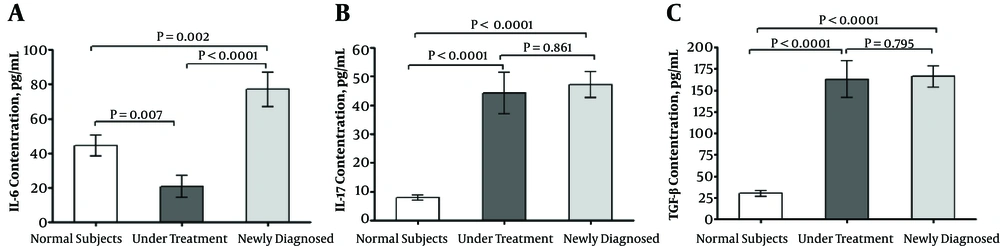
ELISA method was used to evaluate the plasma levels of IL-6 among the ND patients in comparison to the healthy subjects. The plasma expression of IL-6 among the ND patients was higher than the healthy subjects (P = 0.002) and the UT patients (P < 0.0001). The plasma level of IL-6 was decreased among the UT patients in comparison to the healthy subjects (P = 0.007) (Figure 1A). Moreover, IL-17 was significantly overexpressed among the ND (P < 0.0001) and UT (P < 0.0001) patients compared with the healthy subjects. However, no significant difference was observed between the two groups of the patients (P = 0.861) (Figure 1B). Similarly, the expression of TGF-β was not different between the ND and UT patients (P = 0.795). However, it was significantly decreased among the healthy subjects compared with the ND (P < 0.0001) and UT (P < 0.0001) patients (Figure 1C).
4.2. The Segregation Value of Cytokines Between Patients and Normal Subjects
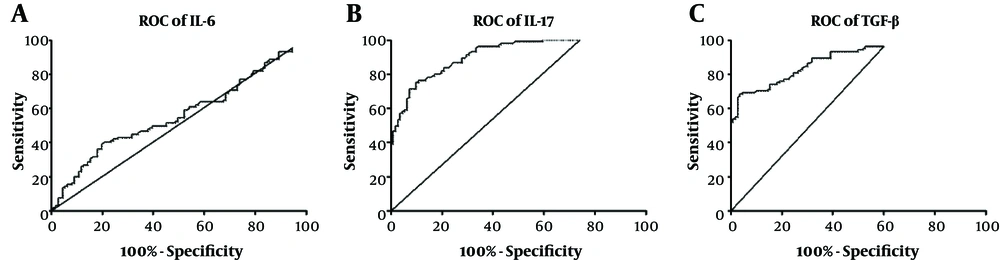
Receiver operating characteristic (ROC) curve analysis was used to assess the diagnostic utility of the evaluated cytokines to distinguish between infected and non-infected samples. Area under the curve (AUC) for IL-6 was 0.5598 (95% CI: 0.4823 - 0.6373; P = 0.129). Setting the optimal cut-off value at 31.95 (likelihood ratio = 1.14) gave a sensitivity of 51.43% (95% CI: 41.47% - 61.30%) and a specificity of 54.95% (95% CI: 45.22% - 64.41%) (Figure 2A). AUC for IL-17 was 0.9148 (95% CI: 0.8798 - 0.9497; P < 0.0001). Setting the optimal cut-off value at 14.05 (likelihood ratio = 3.95) resulted in a sensitivity of 81.90% (95% CI: 73.19% - 88.74%) and a specificity of 79.28% (95% CI: 70.55% - 86.39%) (Figure 2B). Moreover, AUC for TGF-β was 0.8877 (95% CI: 0.8440 - 0.9315; P < 0.0001). Setting the optimal cut-off value at 51.20 (likelihood ratio = 3.03) lead to a sensitivity of 82.69% (95% CI: 74.03% - 89.41%) and a specificity of 72.73% (95% CI: 63.41% - 80.78%) (Figure 2C).
4.3. The Segregation Value of IL-6 Between Newly Diagnosed and Under Treatment Patients
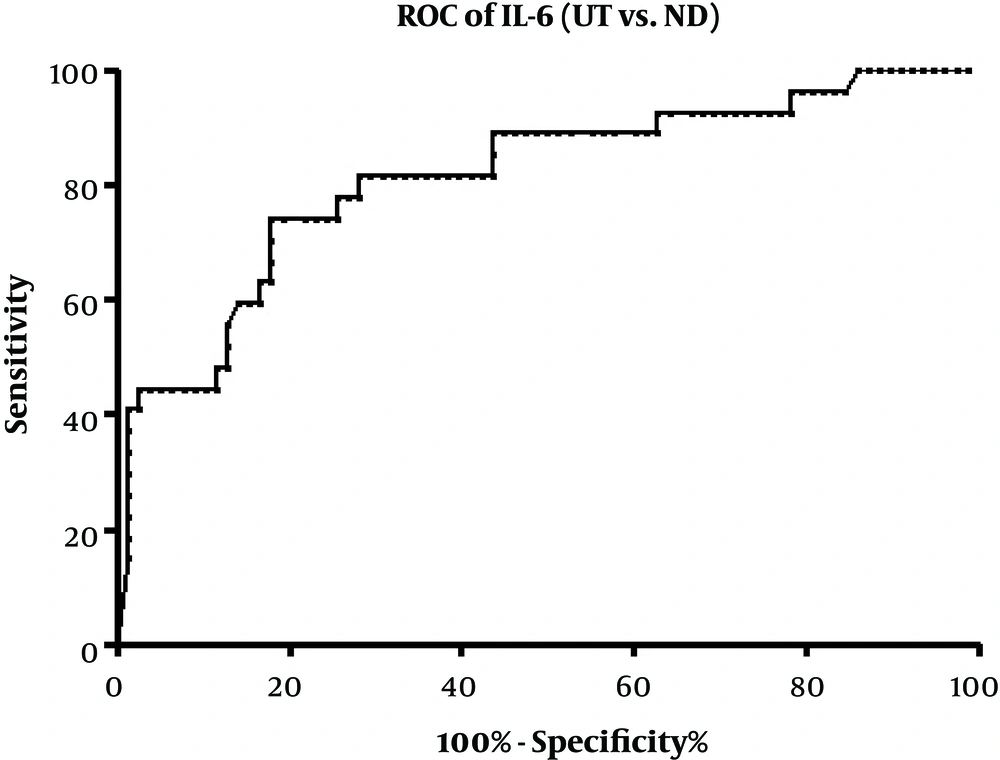
We also used ROC curve analysis to investigate the diagnostic utility of IL-6 to distinguish between the ND and UT patients, which was the only altered cytokine following the treatment. Area under the curve (AUC) for IL-6 was 0.8139 (95% CI: 0.7147 - 0.9130; P < 0.0001). Setting the optimal cut-off value at 23.75 (likelihood ratio = 3.03) gave a sensitivity of 77.78% (95% CI: 57.74% - 91.38%) and a specificity of 74.36% (95% CI: 63.21% - 83.58%) (Figure 3).
ELISA cytokine assay; IL-6 plasma level was significantly higher in newly diagnosed patients with TB (A). IL-17 (B) and TGF-β (C) were significantly increased in both groups of patients with TB. The non-parametric Kruskal-Wallis with Dunn-Bonferroni post hoc test was also used to compare the means of multiple samples. Data of each bar demonstrates mean ± SE. P values lower than 0.05 were considered statistically significant. SE, standard error; TB, Tuberculosis.
ROC curve analyses (patients with TB vs. healthy subjects); area under the curve (AUC) for IL-6 was 0.5598 (P = 0.129). Setting the optimal cut-off value at 31.95 gave a sensitivity of 51.43% and a specificity of 54.95% (A). AUC for IL-17 was 0.9148 (P < 0.0001). Setting the optimal cut-off value at 14.05 resulted in a sensitivity of 81.90% and a specificity of 79.28% (B). AUC for TGF-β was 0.8877 (P < 0.0001). Setting the optimal cut-off value at 51.20 lead in a sensitivity of 82.69% and a specificity of 72.73% (C).
5. Discussion
According to the growing burden of tuberculosis (TB) on public health in developing countries, early diagnosis and timely interventions are indispensable to effectively control TB (6, 25). Nowadays, conventional diagnostic tests, such as microscopic examination and culture of acid-fast MTB organisms, are replaced by T-cell-based assays such as interferon-gamma release assay (IGRA) (7), which is also known as QuantiFERON-TB Gold test (8, 26). However, IGRA is an expensive and complicated assay, which is not also capable of distinguishing active TB infections from latent (10). Moreover, the evaluation of IFN-γ in the plasma (as a probable alternative for IGRA) is not trusted due to the scant amounts of detectable secreted IFN-γ (27). Therefore, there is an urgent need for more convenient and inexpensive TB biomarkers (11). Cytokines are major regulators of immune responses, which are secreted by immune cells and have definite roles in determining the fate of host-pathogen interaction (12, 13). Cytokine levels are altered in different states of the disease and could be of diagnostic value (13). Several studies have introduced cytokines and chemokines as potent TB biomarkers, such as IFN-γ (16), IP-10 (28), MCP-2 (29), TGF-β (14), IL-10 (30), IL-6 (31), and TNF-α (32). Therefore, cytokines can be introduced as potential diagnostic and prognostic TB biomarkers (14). However, previous studies have not thoroughly addressed the differences between active and latent TB infection, which is of great importance in determining the prescribed treatment strategies (33). Hence, we evaluated the plasma expression of IL-17, IL-6, and TGF-β among newly diagnosed TB patients in comparison to healthy subjects and analyzed the diagnostic utility of each cytokine to introduce a novel TB biomarker which may distinguish active TB infection.
The pro-inflammatory IL-6 is secreted by T cells and macrophages (34) and is involved in the pathogenesis of TB by stimulating the secretion of IFN-γ (18). It has been introduced in several studies as a potent TB biomarker (35). However, no clinical studies have confirmed the ability of IL-6 to distinguish the patients with active TB. In the current study, we showed that the plasma levels of IL-6 among newly diagnosed patients with TB were overexpressed but not statistically significant in comparison to the healthy subjects. We also used ROC curve analysis to assess the diagnostic utility of IL-6. In this regard, our findings reveal that the AUC for IL-6 has not an acceptable segregation value in distinguishing patients with TB from the heathy subjects. Moreover, the most suitable cut-off value for IL-6 did not give proper sensitivity and specificity. Therefore, IL-6 may not be introduced as a suitable TB biomarker to distinguish the disease. On the other hand, IL-6 was the only cytokine which was different between the two groups of the patients. ROC curve analysis to assess the diagnostic utility of IL-6 between the ND and UT patients gave an acceptable AUC with proper sensitivity and specificity. Therefore, IL-6 could be introduced as a suitable marker in distinguishing active state from latent and also monitoring the treatment efficiency.
IL-17 is a recently specified pro-inflammatory cytokine, which is known for its role in immune responses against extracellular pathogens (19). However, the role of IL-17 in MTB infection has been controversial (20). It has been reported that IL-17+CD4+ T cells constitute a higher population in healthy subjects compared to the patients with active and latent TB (36). On the other hand, IL-17 was reported to be downregulated in the PBMCs of vaccinated normal subjects in comparison to patients with active TB (37). However, no study has evaluated the diagnostic value of IL-17 plasma alterations in discrimination of patients with active TB. In the present study, we showed that IL-17 was significantly overexpressed among newly diagnosed patients with TB. ROC curve analysis results showed an AUC of 0.9148, which is an optimum segregation value with acceptable sensitivity and quite high specificity.
TGF-β is a multifunctional cytokine, which is produced by T cells, B cells, and myeloid cells (21), and may be involved in the suppression of T cell responses, macrophage deactivation, and tissue injury initiation and propagation (22). It has been reported that the production of TGF-β was augmented by blood monocytes from patients with active TB (38). TGF-β is also increased in the HIV and pulmonary TB infections, which highlights the role of this cytokine in the propagation of TB-associated disorders (39). In the present study, we reported that TGF-β was significantly increased among newly diagnosed patients with TB. ROC curve analysis showed an optimum segregation value (AUC = 0.8877) with quite high sensitivity and acceptable specificity. Altogether, IL-17 and TGF-β plasma levels may be introduced as candidate biomarkers in the diagnosis of patients with active TB.
5.1. Limitations
The present study had several limitations. The most important limitation of our research was about comparing the cytokine results of TB patients with normal subjects without any pulmonary diseases and not including patient groups suffering from other pulmonary disorders. Although we did not generalize the introduced biomarkers to distinguish TB from other diseases and we aimed to specifically segregate the patients with active TB from latent TB, we suggest designing further studies on patients suffering from non-TB pulmonary disorders to confirm or reject the specificity of our introduced biomarkers.
5.2. Conclusions
In conclusion, the expression of IL-6 was higher in newly diagnosed patients with TB. Therefore, IL-6 could be introduced as a suitable marker in distinguishing active TB state from latent and also monitoring the treatment efficiency. IL-17 and TGF-β cytokines were significantly overexpressed in newly diagnosed patients with TB. Although IL-17 is more specific in distinguishing active TB infection and TGF-β represented a higher sensitivity, it is suggested that multiple cytokine assay should be conducted to have a better diagnostic performance rather than individual cytokine evaluation.