1. Background
Mucormycosis is an opportunistic fungal infection caused by members of the Mucorales order. The infection is less common than candidiasis and aspergillosis; however, the incidence of mucormycosis has been increased steadily during the last decades (1, 2). Some of the most important predisposing conditions for mucormycosis are prolonged neutropenia, corticosteroid and deferoxamine therapy, diabetic ketoacidosis, hematologic malignancies, aplastic anemia, myelodysplastic syndrome, solid organ or hematopoietic stem cell transplantation, penetrating trauma, and burns (3, 4). Although mucormycosis in developed counties is mostly reported in patients with underlying diseases such as hematological malignancies, chemotherapy, and bone marrow transplantation, uncontrolled diabetes mellitus is the main predisposing factor for mucormycosis in developing countries (5, 6). In high-risk patients, 5% - 12% of the invasive fungal infections are usually caused by Mucorales; however, the higher rates (20% - 44%) have also been reported (7, 8).
Based on the anatomical location, mucormycosis falls into six clinical forms, including rhino-cerebral (48%), pulmonary (24%), cutaneous (19%), gastrointestinal (7%), disseminated (3%), and uncommon or rare forms (6%) such as endocarditis, osteomyelitis, and peritonitis (3, 7, 9). Rhizopus species have usually been recovered from mucormycosis specimens whereas other Mucorales, including Mucor spp., Cunninghamella spp., Apophysomyces spp., Saksenaea spp., Rhizomucor spp., Syncephalastrum spp., and Cokeromyces spp. are less common etiologic agents of infection (4, 10).
Laboratory diagnosis is based on microscopic examination and culture of clinical specimens. Polymerase chain reaction (PCR) and PCR-based sequencing have been used for laboratory diagnosis of the infection and species identification of the causative agents (4, 11). Although direct microscopy of the involved tissue, as well as culture and microscopic observation of reproductive structures, are the most common methods in the clinical laboratory, application of molecular methods can improve the management of patients with mucormycosis (12).
Here, the clinical and laboratory data of 25 cases with mucormycosis was reported whose clinical specimens have been referred to a Medical Mycology Laboratory in Tehran.
2. Objectives
In order to provide accurate microbial epidemiology data, sequence-based identification of the causative fungi was also performed.
3. Methods
3.1. Specimen Collection and Processing
A set of 25 clinical specimens or previously isolated fungi from patients with mucormycosis were included in this study (Table 1). Of them, 15 cases were collected from 2009 to 2013 and 10 cases were obtained from January 2014 to October 2016. These specimens were collected in Medical Mycology Laboratory, Tehran University of Medical Sciences, Tehran, Iran. Demographic data and clinical histories, including underlying diseases, biopsy site, antifungal therapies, and the outcome of the disease were collected from the laboratory and hospital records. This study was approved by the Ethics Committee of Tehran University of Medical Sciences.
| No. | Age/Sex | Province of Residence | Type of Infection | Biopsy Site | Underlying Disease | Direct Examination | Culture | Treatment | Outcome | Morphological Identification | Molecular Identification | Accession Number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/57 | Alborz | RC | Paranasal sinus | Diabetes | Positive | Negative | AmB | Expired | NG | NP | NP |
| 2 | F/49 | Markazi | RC | Paranasal sinus/eye | Diabetes | Positive | Positive | AmB | Expired | Rhizopus spp. | R. oryzae | MF850346 |
| 3 | M/63 | Alborz | RC | Nasal cavity | Tuberculosis | Positive | Positive | NA | Expired | Rhizopus spp. | R. oryzae | MF850347 |
| 4 | M/24 | Tehran | RC | Paranasal sinus | ALL/thalassemia major/diabetes | Positive | Positive | AmB | Expired | Rhizopus spp. | R. oryzae | MF850348 |
| 5 | F/21 | Lorestan | RC | Paranasal sinus | AML-M3 | Positive | Positive | NA | Cured | Rhizopus spp. | R. oryzae | MF850349 |
| 6 | F/33 | Tehran | RC | Paranasal sinus | ALL | Positive | Positive | AmB | Expired | Rhizopus spp. | R. oryzae | MF850350 |
| 7 | F/26 | Tehran | RC | Paranasal sinus /eye | DKA | Positive | Negative | AmB | Expired | NG | NP | NP |
| 8 | F/48 | Isfahan | RC | Nasal cavity | Diabetes | Positive | Positive | AmB | Expired | Rhizopus spp. | R. oryzae | MF850352 |
| 9 | F/10 | Alborz | RC | Jaw | Diabetes | Positive | Positive | AmB | Cured | Rhizopus spp. | R. oryzae | MF850353 |
| 10 | M/28 | Tehran | RC | Nasal cavity | Addiction | Positive | Positive | NA | Cured | Rhizopus spp. | R. oryzae | MF850354 |
| 11 | F/69 | Tehran | RC | Paranasal sinus | Wegner’s syndrome | Positive | Negative | NA | Expired | NG | NP | NP |
| 12 | M/24 | Tehran | RC | Paranasal sinus/nasal cavity | Aplastic anemia | Positive | Positive | AmB/PSC | Cured | Rhizopus spp. | R. oryzae | MF850356 |
| 13 | M/57 | Tehran | RC | Paranasal sinus | Diabetes | Positive | Negative | AmB | Cured | NG | NP | NP |
| 14 | F/42 | Tehran | RC | Paranasal sinus | PNH | Positive | Positive | AmB | Expired | Rhizopus spp. | R. oryzae | MF850358 |
| 15 | F/52 | Tehran | RC | Paranasal sinus | Diabetes | Positive | Positive | AmB | Expired | Rhizopus spp. | R. oryzae | MF850359 |
| 16 | F/69 | Tehran | RC | Paranasal sinus | Diabetes | Positive | Negative | AmB | Cured | NG | NP | NP |
| 17 | M/40 | Qom | RC | Paranasal sinus | Diabetes | Positive | Positive | AmB | Cured | Rhizopus spp. | R. oryzae | MF850355 |
| 18 | F/63 | Alborz | RC | Paranasal sinus | Diabetes | Positive | Negative | NA | NA | NG | NP | NP |
| 19 | F/64 | West Azarbaijan | RC | Paranasal sinus | Metastatic colon cancer | Positive | Negative | NA | Expired | NG | NP | NP |
| 20 | F/54 | Alborz | P | BAL | Diabetes/liver transplant | Positive | Positive | NA | Expired | Rhizopus spp. | R. oryzae | MF850351 |
| 21 | M/67 | Tehran | RC | Paranasal sinus/nasal cavity | CLL/diabetes | Positive | Positive | AmB | Expired | Rhizopus spp. | R. oryzae | MF850357 |
| 22 | M/43 | Alborz | RC | Nasal cavity | Diabetes | Positive | Positive | AmB | Cured | Rhizopus spp. | R. oryzae | MF850360 |
| 23 | F/40 | Khuzestan | RC | Nasal cavity | Diabetes/kidney transplant | Positive | Negative | NA | NA | NG | NP | NP |
| 24 | M/67 | Tehran | RC | Paranasal sinus | Diabetes | Positive | Negative | NA | NA | NG | NP | NP |
| 25 | F/69 | East Azerbaijan | RC | Nasopharynx | Diabetes | Positive | Negative | AmB | Cured | NG | NP | NP |
Abbreviations: ALL, acute lymphoblastic leukemia; AmB, amphotericin B; AML, acute myeloblastic leukemia; BAL, bronchoalveolar lavage; CLL, chronic lymphoblastic leukemia; DKA, diabetic ketoacidosis; F, female; M, male; NA, not available; NG, no growth; NP, not performed; P, pulmonary; PNH, paroxysmal nocturnal hemoglobinuria; PSC, posaconazole; RC, rhinocerebral.
Microscopic examination of these specimens was performed by KOH (10% - 20% potassium hydroxide) wet mount. Furthermore, a part of all the specimens was cultured on Sabouraud dextrose agar medium (SDA, Himedia, India) and incubated at 30°C for initial three days and then at room temperature (23 - 26°C)
3.2. Morphological and Molecular Identification
Slide culture was prepared and morphological characteristics of the diagnostic structures (sporangiophores, columella, sporangium, sporangiospore, and rhyzoied) were imaged and analyzed using an optical microscope (Olympus BX41, Olympus Corporation, Japan) and OLYSIA Bio Report software.
For molecular identification, all isolates were sub-cultured on SDA medium and their DNAs were extracted and purified as described previously (13). The entire internal transcribed spacer (ITS) region (ITS1-5.8S rDNA-ITS2) was amplified by using universal primers ITS1 (5'-TCC GTA GGT GAA CCT GCG G-3') and ITS4 (5'-TCC TCC GCT TAT TGA TAT GC-3') in PCR conditions described previously (14). The purity of PCR-amplified products was checked by electrophoresis on 1.5% agarose gel and all PCR-amplicons were sent for Sanger sequencing (Bioneer company, South Korea). Speciation of isolates was done by comparing the sequences with GenBank data using basic local alignment search tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST/) and all sequences were deposited in GenBank under the accession numbers of MF850346 to MF850360.
4. Results
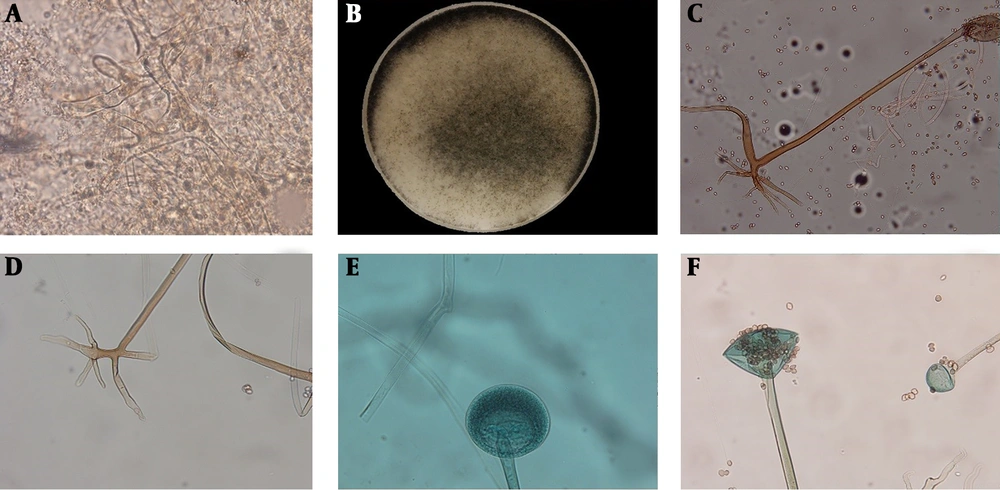
In direct examination of all the specimens, broad non-septate hyphal elements were observed (Figure 1). Females (n = 17, 68%) were more affected than males (n = 8, 32%). The age of the patients ranged from 10 to 69 years (mean = 47.16 ± 17.4 years). All of the patients were immunocompromised and diabetes mellitus was the most common predisposing factor (n = 17, 68%), followed by solid organ transplant and hematological disorder/malignancy such as leukemia, each of them contained two patients. Rhino-cerebral infection (n = 24) was the most common type of disease, also one case of pulmonary mucormycosis was recorded in this study (Table 1). Thirteen patients out of 22 patients (59.09%) were expired (according to available data. The data of some cases were missing) (Table 1).
Broad aseptate hyphal elements with right branching in direct examination of a clinical specimen (A), macroscopic view of grown colony on Sabouraud dextrose agar medium (B), the microscopic features of sporangiophore (C), rhizoids (D), and immature- (E) and mature- (F) sporangium in slide cultures of Rhizopus oryzae (× 400)
The culture was positive for 15 specimens and the remaining specimens yielded no fungal growth even after numerous repeated cultures. The following morphologic characteristics were obtained in slide cultures by using OLYSIA Bio Report software: (i) single or often paired sporangiophores, mostly unbranched, brownish and between 400 µm to 2 mm in length, (ii) sporangiums mostly spherical to conical, brownish-gray and between 50 - 250 µm in diameter and (iii) spherical to conical columellas and branched rhizoid with 100 - 250 µm in length. The isolated fungi were morphologically identified as Rhizopus spp. and further identification was not feasible at the species level (Figure 1). However, all the isolates were identified at the species level as R. oryzae using the molecular method (Table 1).
5. Discussion
Mucormycosis is an opportunistic fungal infection that was first reported in 1885 (4). Over the past two decades, the incidence of mucormycosis has dramatically increased, as a result of an increase in the population at risk (6). Despite antifungal therapy and aggressive surgical interventions, the mortality rate associated with mucormycosis is > 47% emphasizing the importance of this disease (15). In the present study, we analyzed the clinical data of 25 Iranian cases with mucormycosis and the causative agents were identified through morphological and molecular procedures.
In our study, the mean age of the patients was 47 years similar to some previous reports (range, 40 - 52 years) (16-19). In contrast, Dai et al. (9) and Saegeman et al. (7) have reported the mean ages of 58.8 and 60 years, respectively. Although mucormycosis has been reported to be more common in males compared with females in most of the published series (1, 3, 4, 7, 9, 16, 20), in the present study, females (68%) were more affected than males (32%), which is in accordance with reports by Komur et al. (21) from Turkey and Al Akhrass et al. (22) from the United States of America. These differences could be due to variations in study populations.
Rhinocerebral mucormycosis is the most common clinical form of this infection (4, 6, 17, 21, 23), which is in agreement with our results. In our study, similar to a report by Roden et al. (17) from the United States of America, rhinocerebral mucormycosis with or without orbital involvement was the major manifestation in diabetic patients. These findings are inconsistent with the results of Zaki et al. (3) from Egypt, which demonstrated pulmonary mucormycosis as the major manifestation in diabetic patients. In this report, diabetes mellitus (68%) was the common risk factor for mucormycosis, which supports other studies (3, 4, 20, 24), as expected in tropical and developing countries (3, 6). However, in some other studies, hematologic malignancy has been reported as the most common predisposing factor for mucormycosis (21, 25).
Recovery of mucoralean fungi from clinical specimens has been found problematic because of negative culture results, which could be due to the tissue processing prior to culture (26, 27) as well as initiation of therapy prior to specimen collection, which result in a negative culture. In the present study, the positive culture was obtained in 60% of the cases, which is similar to the data reported in a review of 929 mucormycosis cases (1940 - 2003) with 50% positive cultures (17). However, Zaki et al. (3), reported a recovery rate of 100%. These discrepancies could be due to the difference in the procedure of specimen processing.
According to morphological characteristics, all isolates were identified as Rhizopus spp. and the results of molecular identification were 100% confirmed at the genus level, similar to Alvarez et al. (28). Therefore, conventional morphological methods, which are available in all Medical Mycology Laboratories could be trusted at the genus level at least for the Rhizopus as most common genus. However, these procedures are time-consuming and need high levels of expertise, especially in the case of uncommon species. Also, accurate identification may not be achieved for all genera as Yang et al. (15) reported a lower concordance rate (58.3%) between conventional and molecular identifications. Similarly, Kontoyiannis et al. (18) showed a 20% error rate for conventional identification in comparison to PCR-sequencing results. Therefore, simultaneous application of both morphologic and molecular procedures could be of great importance to provide accurate results.
Members of the genus Rhizopus are the principal causes of mucormycosis (1, 4, 7, 22). Although 10 of 25 specimens yielded no colonies in this study, all 15 grown isolates were identified as R. oryzae, which implies a prevalence of at least 60% for this species as the most common agent in this study. Similar results could be found in studies by Bala et al. and Alvarez et al. (4, 28). However, others (3, 29, 30) reported species other than R. oryzae as the prevailing etiology of mucormycosis. These differences emphasize the need for precise identification in all cases of mucormycosis.
Finally, the present study used sequence-based method for precise identification of the isolates, though, the results could be beneficial for a better understanding of the microbial etiology of mucormycosis. However, the number of the patients were limited and positive culture was not obtained for some cases, which are some shortcomings of this study.
5.1. Conclusions
It could be concluded that the spectrum of fungal etiology of mucormycosis could differ based on the study populations and geographic locations. Regarding full agreement between the results of morphologic and molecular methods in our study, conventional procedures could be trusted at genus level at least for Rhizopus species; however, application of molecular methods is recommended due to their high identification power.