1. Background
Regular and prolonged blood transfusion in patients with inherited disorders of hemoglobin (IDH), particularly thalassemia, exposes them to blood-transmitted infections, including viral hepatitis (1). The implementation of effective vaccination against hepatitis B and screening of blood and blood products for hepatitis C since the 1990s has resulted in a remarkable reduction in hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. However, post-transfusion transmission of HCV has remained a significant health concern in thalassemia patients (2, 3).
Chronic post-transfusion hepatitis B and C can lead to hepatocellular necrosis, fibrosis, and cirrhosis in patients with thalassemia, which has been accepted as a significant cause of morbidity and mortality in these patients (4). In Iran, since 1996, the routine screening for the main blood-borne diseases has been conducted among blood donors, resulting in a significant decrease in the HCV incidence in thalassemia patients (5). Previous studies in Iran reported the seroprevalence of HCV in beta-thalassemia patients in a wide range of 7% - 34% and the prevalence of HBV from 0.3% to 7% (6-8).
Sistan and Baluchistan province is located in the southeast of Iran, bordering with Afghanistan and Pakistan. This province with more than 2500 cases of thalassemia has one of the highest prevalence rates of thalassemia in Iran (9). Moreover, this province has a high prevalence of HBV infection with more than 3% prevalence of hepatitis B surface antigen (HBsAg) in the general population (10).
2. Objectives
Due to the lack of sufficient reported data from Sistan and Baluchistan province, we conducted this multicenter study on the epidemiology of HBV and HCV infections in patients with IDH in Sistan and Baluchistan.
3. Methods
3.1. Study Population
In this retrospective study, 2391 patients who referred to the thalassemia clinics in Sistan and Baluchistan province until April 2016 were initially enrolled. The inclusion criteria included: (1) Patients who had thalassemia or other inherited disorders of hemoglobin, (2) patients who had permanent residence (more than five years) in Sistan and Baluchistan province, (3) patients who had transfusion-dependent IDH with the need of more than once a year transfusion of the packed cell, and (4) patients with available results for the detection of anti-HCV antibody (HCV Ab) and HBsAg. The Ethics Committee of the Baqiyatallah Research Center for Gastroenterology and Liver Diseases approved the study design. All study participants provided informed consent. The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
3.2. Laboratory Studies
The detection of HBsAg and HCV Ab was performed in the Iranian Blood Transfusion Organization, Zahedan, Iran. For the detection of HBsAg, patients’ sera were tested using Enzygnost HBsAg 6.0 (Siemens). Patients’ sera were tested using EIAgen HCV Ab (v.4) kit (Adaltis) for the evaluation of HCV Ab. In a proportion of patients with a positive result for HCV Ab, the HCV RNA detection and HCV genotyping were performed using valid methods in a private diagnostic laboratory. Moreover, the data of hepatitis B surface antibody (HBsAb) were extracted from patients’ records.
3.3. Statistical Analysis
Categorical variables were expressed as frequencies and percentages. Continuous variables were expressed as means ± standard deviation (SD). Comparisons between groups were made using Student’s t test for continuous variables and Chi-square or Fisher’s exact test, when appropriate, for categorical variables. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 20.0 (IBM SPSS). Statistical graphs were generated using GraphPad Prism version 6.0 (GraphPad Software).
4. Results
4.1. Patients’ Characteristics
Among 2391 registered patients with IDH, 2387 cases with a mean age of 12.7 years were included in the final analysis and four patients were excluded because of unavailable data for HBsAg. Most of the patients were residents of Zahedan city (28.1%) and suffered beta-thalassemia major (99.2%). Moreover, 9.2% of the patients had a history of splenectomy and 2.2% were diabetic. The patients’ characteristics are summarized in Table 1.
| Variables | Values |
|---|---|
| Sex | |
| Male | 1271 (53.2) |
| Female | 1116 (46.8) |
| Total | 2387 |
| Age, y | |
| Mean ± SD | 12.7 ± 7.7 |
| Range (min - max) | 1 - 50 |
| Total | 2376 |
| City of residence | |
| Chabahar | 452 (18.9) |
| Iranshahr | 405 (17.0) |
| Khash | 148 (6.2) |
| Nikshahr | 136 (5.7) |
| Saravan | 391 (16.4) |
| Zabol | 184 (7.7) |
| Zahedan | 671 (28.1) |
| Total | 2387 |
| Type of inherited disorders of hemoglobin | |
| Beta-thalassemia major | 2249 (99.2) |
| Beta-thalassemia intermedia | 5 (0.2) |
| Others | 14 (0.6) |
| Total | 2268 |
| Splenectomy | |
| Yes | 218 (9.2) |
| No | 2146 (90.8) |
| Total | 2364 |
| Diabetes | |
| Yes | 36 (2.2) |
| No | 1614 (97.8) |
| Total | 1650 |
aValues are expressed as No. (%) unless otherwise indicated.
4.2. Hepatitis C Virus in Patients with Inherited Disorders of Hemoglobin
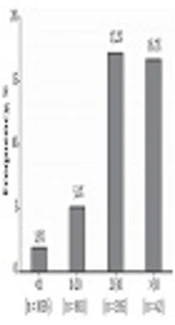
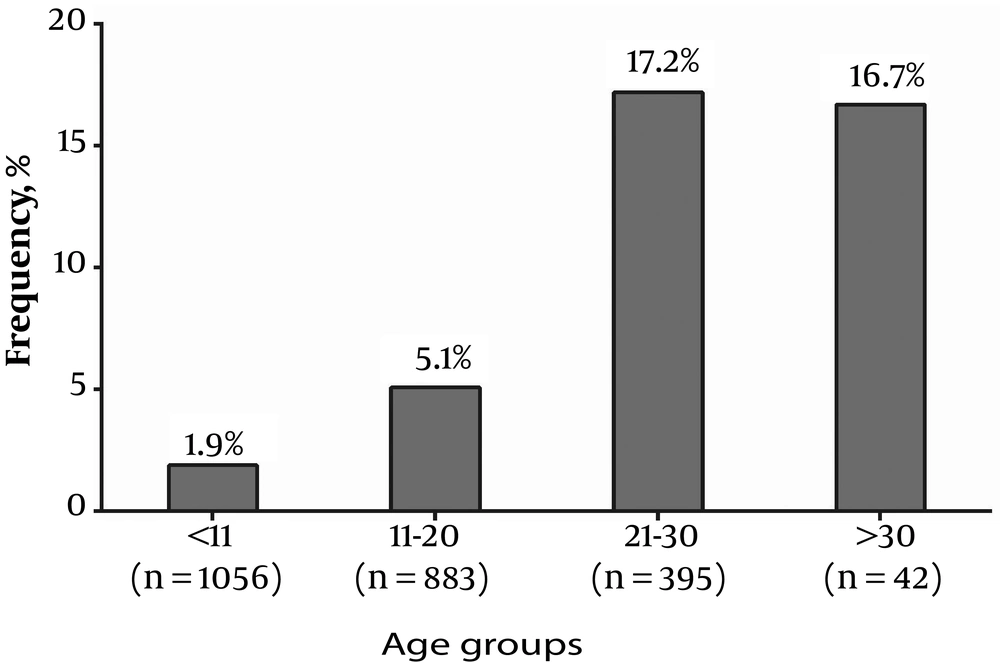
Out of 2387 patients with IDH, 140 (5.87%: 95% CI = 5.0% - 6.89%) were found to be positive for HCV Ab. The rate of positivity for HCV Ab was 8.7%, 8.64%, 7.3%, 4.05%, 3.32%, 2.94%, and 2.47% in Zabol, Zahedan, Chabahar, Khash, Saravan, Nikshahr, and Iranshahr, respectively (P < 0.001). Gender and type of IDH did not influence the detection rate of HCV Ab (P > 0.05) while the age of the patients with IDH was significantly associated with the rate of HCV Ab detection (P < 0.05) (Figure 1). Furthermore, IDH patients with HCV Ab were splenectomized more frequently than patients without HCV Ab (30.2% vs. 7.9%; P < 0.001). In addition, HCV-seropositive patients were more diabetic than HCV-seronegative patients (6.3% vs. 2.0%; P = 0.026).
Among 101 patients with positive results for HCV Ab who were tested for HCV RNA by RT-PCR, 53 (52.5%; 95% CI = 42.8% - 61.9%) had HCV RNA in their serum samples. Moreover, 40 patients had results for HCV genotyping including 29 (72.5%) HCV genotype 3 and 11 (27.5%) HCV genotype 1.
4.3. Hepatitis B Virus in Patients with Inherited Disorders of Hemoglobin
Among 2387 patients with IDH, 7 (0.29%; 95% CI = 0.14% - 0.6%) were found to have positive results for HBsAg. Out of seven patients with HBsAg, three (42.9%) were from Iranshahr, five (71.4%) were females, five (71.4%) were younger than 20-years-old, and two (28.6%) were positive for HCV Ab. Out of 1283 IDH patients with data for the detection of HBsAb, 1248 (97.27%; 95%CI = 96.23% - 98.03%) had positive results for HBsAb.
5. Discussion
This study investigated the prevalence of hepatitis B and hepatitis C seromarkers in a large cohort of 2387 patients with IDH in Sistan and Baluchistan province and found 5.87% HCV seroprevalence and 0.29% HBV seroprevalence.
In Iran, HCV infection in thalassemia patients is more frequently observed than in the general population mainly due to blood transfusion before 1996 when the donated blood was not screened for HCV Ab (8). In a meta-analysis, the prevalence of HCV Ab was between 2% and 32% among thalassemia patients in different provinces of Iran with a pooled seroprevalence of 18% (8). The first study on the seroprevalence of HCV in thalassemia patients in Sistan and Baluchistan province was conducted by Sanei Moghaddam et al. (11) in 2004 with the observation of 13.5% positivity for HCV Ab in thalassemia. The implementation of screening for HCV Ab in blood donations since 1996 has had a significant impact on lowering the rate of transfusion-transmitted HCV infection among thalassemia patients. The findings of the present study confirm the latter fact with the observation of a drop from 17.2% HCV seroprevalence in thalassemia patients aged 21 - 30 (born before 1996) to 5.1% in thalassemia patients aged 11 - 20 (born after 1996). However, the HCV seroprevalence was high even in thalassemia patients born after 1996 with 5.1% in patients aged 11 - 20 and 1.9% in patients aged < 11, which can be because of nosocomial transmission (12). Another two studies from Zabol city showed 8.5% and 10% HCV seroprevalence, which is consistent with 8.7% HCV seroprevalence among participants of the present study from Zabol city (13, 14). In the current study, HCV RNA was detected in 53% of the HCV-seropositive IDH patients with available data for the assessment of HCV RNA. In comparison with 25% spontaneous clearance found in most of the previous studies (15, 16), the observation of 47% HCV-seropositive IDH patients with negative results for HCV RNA should not be reliable and can be caused by the fact that some of the patients were treated for HCV infection previously or as a result of false positive result in HCV Ab testing by ELISA. In this study, 72.5% of the patients were infected with HCV genotype 3 and the remaining with HCV genotype 1. In Iran, HCV genotype 1 is the most prevalent HCV isolate (17, 18). Moreover, HCV genotype 1 was the most isolated genotype from Iranian thalassemia patients with HCV infection (19). However, a previous study in Sistan and Baluchistan found HCV genotype 3 as the dominant genotype in children and adolescents with thalassemia major (20).
In Iran, the prevalence of HBsAg is less than 2%; however, in Golestan and Sistan and Baluchistan provinces, the HBV prevalence has been reported to be more than 2% (10). It has been estimated that 3.38% of the general population of Sistan and Baluchistan are HBsAg-positive (21). In this study, the prevalence of HBsAg in patients with IDH in Sistan and Baluchistan province was less than 0.5%, which is unexpectedly much lower than the prevalence in the general population of Sistan and Baluchistan. The low prevalence of HBV infection in Iranian patients with IDH was reported previously, as well (1, 6). The low seroprevalence of HBV in thalassemia patients can be because of HBV vaccination of infants and thalassemia patients as high-risk groups for transmission of HBV and increased mortality of patients with IDH and HBV infection.
In recent years, the discovery of HCV direct-acting antiviral agents (DAA) has changed the treatment of HCV infection dramatically (22-24). The previous Pegylated-interferon and Ribavirin combination therapy was inefficient and accompanied by many side-effects while the new DAA regimens are efficient and can be used in patients with thalassemia safely (25, 26). With these new HCV antiviral regimens, there is a great hope to eliminate HCV infection globally by 2030 (27).
This study was limited by the fact that cases with positive results for HCV Ab or HBsAg were not evaluated with an additional method for the confirmation of the positive results. Moreover, HBcAb was not assessed for most patients; so, we excluded this seromarker from this study.
In conclusion, HCV had a high seroprevalence of 5.87% in patients with IDH in Sistan and Baluchistan while hepatitis B had a low seroprevalence of 0.29% that is unusual in a region with more than 3% prevalence of hepatitis B in the general population. Safety measures for blood transfusion have been effective, resulting in decreased HCV seroprevalence in younger patients with IDH. The hepatitis C viremic patients with IDH should be treated with new HCV DAA regimens to prevent the progression of liver diseases in these patients and any residual risks of nosocomial transmission of HCV among patients with IDH.